Volume 12 - Year 2025 - Pages 88-94
DOI: 10.11159/jffhmt.2025.009
Comparative Evaluation of Membrane Contactor and Stripping Device for Ammonia Separation
Jiri Lindovsky1, Katerina Mayerova2, Josef Kalivoda1, Ondrej Kristof2
1Institute of Chemistry and Technology of Environmental Protection, Faculty of Chemistry,
Brno University of Technology, Purkyňova 464/118, 612 00 Brno, Czech Republic
Jiri.Lindovsky@vutbr.cz; Josef.Kalivoda@vut.cz
2Heat Transfer and Fluid Flow Laboratory, Faculty of Mechanical Engineering
Brno University of Technology, Technicka 2896/2, 616 69 Brno, Czech Republic
Katerina.Mayerova@vut.cz; Ondrej.Kristof@vut.cz
Abstract - This study compares two semi-operational devices for ammonia separation from high-ammonia wastewater: a membrane contactor and a stripping device. The membrane contactor utilizes a bundle of hollow-fibre membranes, facilitating the transfer of ammonia nitrogen from the outer shell into an acidic solution inside the fibres. In contrast, the stripping device operates across various temperatures, with ammonia removal assessed by absorption into an acidic solution. Both devices were tested using a model solution simulating high-ammonia liquid digestate from a biogas plant to determine their optimal operating conditions. The pilot experiments evaluated separation efficiency and highlighted each technology's unique advantages and limitations. Our results demonstrate that the membrane contactor achieved the highest efficiency using a KH₂PO₄ solution, long fibres, and elevated temperature, resulting in complete ammonia removal within 240 minutes without pH adjustment, with 50% efficiency at 60 minutes. Further measurements without any condition adjustments resulted in a final separation efficiency of 69 ± 6% after 300 minutes, with 50% efficiency achieved at 181 minutes. Subsequent experiments using a KHSO₄ absorption solution helped to investigate optimal conditions and process kinetics, but the separation efficiency was lower compared to using KH₂PO₄.When using KHSO₄, a maximum efficiency of 93 ± 3% was obtained in 90 minutes after adjusting the pH to around 12. However, while this pH increase enhanced ammonia desorption, the high base consumption makes it economically unfavourable. In comparison, the stripping device achieved its best results at a pH of 10.5, a temperature of 60°C, and an airflow rate of 120 l/min, reaching 85% efficiency within 50 minutes. At a lower pH of 9, the efficiency dropped to 64% over the same time period. These results highlight the stripping device's potential for faster ammonia removal, though efficiency is heavily dependent on pH and airflow conditions.
Keywords: Ammonia separation, Hollow-fiber membranes, Liquid digestate, Stripping device, Wastewater treatment.
© Copyright 2025 Authors - This is an Open Access article published under the Creative Commons Attribution License terms Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2024-09-09
Date Revised: 2024-12-20
Date Accepted: 2025-02-13
Date Published: 2025-03-04
1. Introduction
High ammonia nitrogen concentrations in various types of wastewaters cause significant negative consequences, not only for the environment but also for the quality of life of animals and plants. Liquid effluents (such as digestate) after anaerobic digestion often contain high ammonia concentrations, which can lead to contamination when used as fertilizer in agriculture. The volatilization of NH₃ is the primary factor contributing to its increased concentration in the atmosphere, and several studies have confirmed that 90% of total atmospheric ammonia originates from agriculture [1], [2]. Additionally, applying large amount of digestate to various kinds of soil can cause ground and groundwater contamination [3]. And for this reason, many European countries have legislation that limits the use of these effluents as fertiliser per unit area [4].
Ammonia removing methods most commonly used in wastewater treatment are nitrification/denitrification, or air stripping [5]. However, there are other modern methods for ammonia separation, such as membrane technologies or struvite precipitation, that are not yet fully optimized.
Steam and air stripping of ammonia are confirmed most efficient in wastewaters with higher concentrations than 1 g / l, but various conditions (temperature, pH, flow rate, etc.) can affect final mass transfer [6]. In general, the desorption of ammonia from water solutions is described by the Henry's law and, the distribution diagram of ammonia species, and also other important variables [7]. Provolo et al. [8] reported that temperatures above 40 °C and pH values greater than 9 are crucial to achieve 90% efficiency. Experiments without significant adjustments (at 30 °C and pH 9) achieved an efficiency of 41 % after 10 days. Alternative stripping gases can also be used with comparable stripping efficiency to an air. For example, Serna‑Maza et al. [9] and Bousek et al. [10] used flue gas and biogas for ammonia stripping, achieving 86% efficiency with flue gas and 47% efficiency with biogas after 4 hours. Similar experiments using air reached 96% efficiency, though it's important to note that the temperature was around 70 °C. Another factor that improves mass transfer is increasing the gas-liquid ratio, which accelerate the renewal of gas-liquid interface, leading to a higher concentration gradient [11]. Finally, actual studies show that vacuum ammonia stripping has the potential to reach comparable efficiency without conditions adjustment by lowering the pressure to 20–50 kPa [12]-[14].
On the other hand, membrane technology for ammonia separation is continuously being improved. Hollow-fibre membranes are the most widely utilized for coarse dispersion wastewaters, such as digestate. Gaseous ammonia passes through the pores of the membrane from the external solution into the absorbing acidic solution inside the hollow-fibre [15], [16]. As with stripping, temperature and pH are the main factors affecting ammonia separation into acidic solution, whereby partial pressure is the driving force for mass transfer [17], [18]. Conventional materials for the fabrication of hollow-fibre membranes are polypropylene (PP), polyvinylidene fluoride (PVDF) and polytetrafluoroethylene (PTFE), prepared via the phase inversion process [19], [20]. These materials have the unique feature of hydrophobicity, which allows gas molecules to pass through the pores between two aqueous solutions [18]. Minor parameters influencing mass transfer include the flow rate of both solutions and the concentration of the absorbing solution, as reported [21]. Riaño et al. [22] investigated ammonia capture from biogas plant digestate using PTFE membrane panels and 1 M sulfuric acid as the absorption solution. Their study demonstrated that replacing the absorption solution during the process (50 days) significantly improved ammonia removal efficiency, with the first solution absorbing 57.5% (2. 30.7 % and 3. 20.7 %) of the total ammonia and confirmed that saturation of the absorption solution leads to ammonia re-release into the digestate. Other studies studied optimal conditions (temperature, pH, absorption solution, etc.) for ammonia transport in membranes and removal efficiencies achieved 80–98.5 % [21], [23].
This work presents a comprehensive analysis of the experimental results obtained from semi-operational membrane contactor and stripping devices designed for ammonia recovery from liquid digestate at biogas plants. Initial experiments utilizing model solutions that simulate real digestate have led to the identification of optimal conditions for enhancing ammonia recovery efficiency. This research contributes valuable insights to the existing knowledge on ammonia recovery technologies and provides essential guidance for optimizing operational parameters, facilitating the transition to full-scale applications in biogas plants. The findings pave the way for improved sustainability in biogas production and wastewater management.
2.Methods
All experiments were carried out with model solutions simulating concentrations of ammonia nitrogen in real samples of a fugate (liquid digestate) according to a local biogas plant. Model solutions were prepared using NH4Cl, and the pH was adjusted with the addition of NaOH so the concentration of ammonia nitrogen ranged from 4–7 g / l. For absorption solution, acidic salts KHSO4 and KH2PO4 were on the membrane contactor, and lately, KH2PO4 has been considered a more effective solution for experiments with a stripping device.
The concentration of ammonia in the membrane contactor was measured using the indophenol method, which was calibrated using Snedecor’s F-test. During the stripping process, samples were analyzed using the ammonium cuvette test, with a relative uncertainty ranging from 3.9 to 4.1 mg/L for concentrations between 40 and 180 mg/L. Due to the need for sample dilution, the measurement uncertainty increased further. The temperature and pH were measured using Yokogawa FU-20 and FU-24 sensors, with a standard error of ±0.1 pH units and ±0.5 °C, respectively.
2.1. Experimental set-up
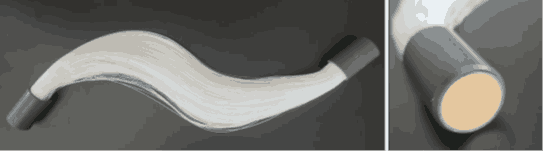
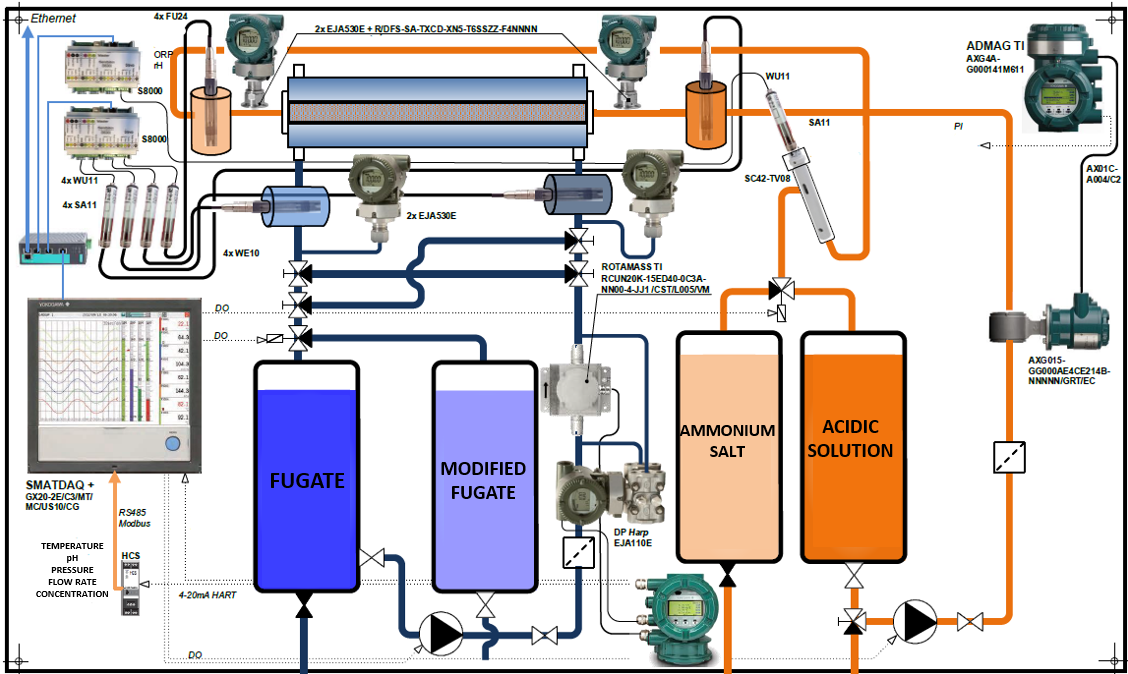
The membrane contactor was constructed with two pipeline routes, where the interfacial contact between phases was realized inside the membrane module. This module featured a bundle of 1380 polypropylene (PP) hollow-fibre membranes, each with a length of 700 mm and pore sizes ranging from 100 to 200 nm. Figure 1 illustrates the hollow‑fibre membranes, including the potting system used to secure and seal the bundle inside the membrane module. The design allowed wastewater to flow outside the fibres, as the coarse particles in the digestate exceed the fibre’s inner diameter, potentially causing clogging. Inside the fibres, an analytical acidic solution was circulated. Sensors were installed on both routes to monitor key operating conditions (pH, temperature, flow rate, pressure), as shown in Figure 2. These sensors were connected to a Memograph M RSG45 datalogger streamlined data analysis. The experiments focused on evaluating the effect of increased pH, temperature and different initial concentrations of ammonia nitrogen. The flow of wastewater was ensured by a diaphragm pump and a centrifugal pump for acidic solution.
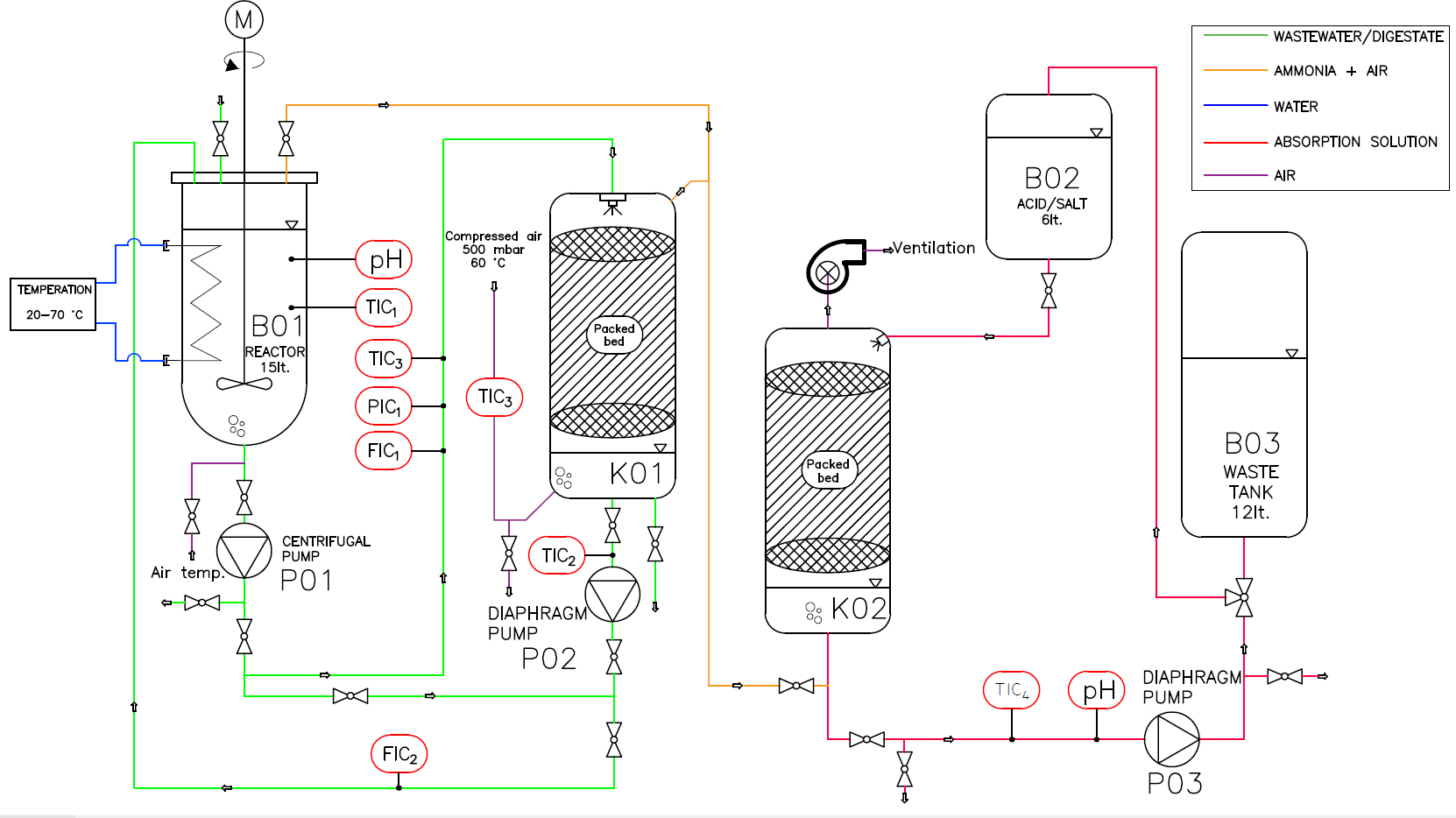
The next tested technology is a stripping device, where wastewater is heated to the required temperature within a glass double-walled batch reactor. Once the wastewater is conditioned, it is introduced into the desorption column, where heated air flows in a countercurrent configuration. The desorbed ammonia is carried with the air into a scrubbing column, where it is captured by an acidic solution flowing countercurrent to the gas stream. The concentration of ammonia nitrogen in the acidic solution is subsequently analyzed using molecular absorption spectroscopy. The sensors and data acquisition setup are identical to those used in the membrane contactor system. A detailed schematic is illustrated in Figure 3.
2.2. Mass transfer kinetics
For the comparison of mass transfer kinetics in both processes, an empirical mass transfer coefficient was evaluated. This process can be described using a pseudo-first-order kinetics model, as the concentration of the absorption solution remains effectively constant. The equation can be expressed as follows:
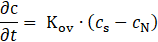
where Kov represents the overall mass transfer coefficient (s–1), cs is the saturation concentration reached when the system attains steady state (g/l), and cN (g/l) is the ammonia nitrogen concentration in absorption solution at time t (s).
After integration, and under the conditions of zero initial concentration and constant saturation concentration of ammonia nitrogen in the receiving solution, the equation 1 is expressed in the following form:
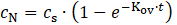
The equation 2 was used to fit experimental data and find overall mass transfer coefficients. Figure illustrates one of the experiments, which helped with determination of mass transfer rate. The pH in this experiment was adjusted to the highest possible level to maximize the mass transfer rate, allowing the entire process to be observed. To fit the experimental data, the BoxLucas1 function in OriginLab was applied, representing the pseudo-first-order kinetics equation. To ensure consistency, all mass transfer coefficients were evaluated using this model across the experiments.
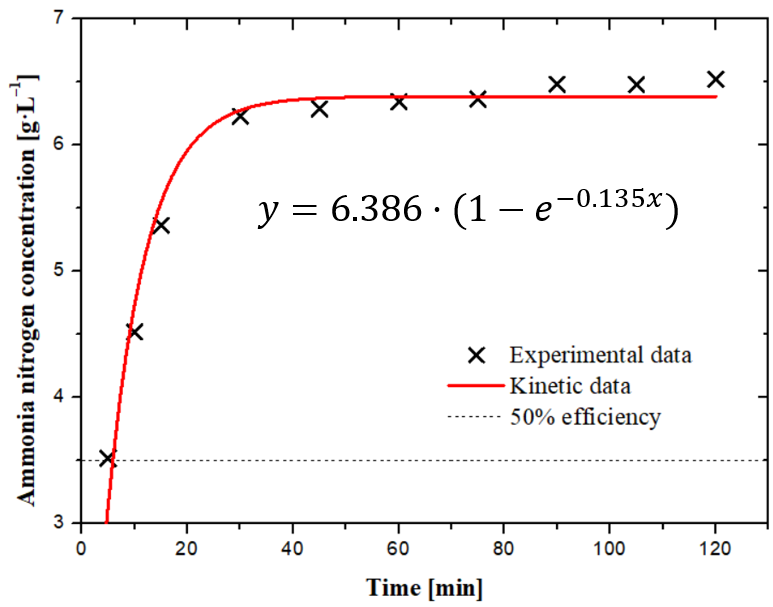
Table 1 : The membrane contactor experiments
|
Experiment |
Absorbent |
T |
pH |
c0 |
50% efficiency |
max. efficiency |
t |
Kov |
|
[°C] |
[-] |
[g / l] |
[min] |
[%] |
[min] |
[s–1] |
||
|
1.1 |
KHSO4 |
34 |
13.68–12.07 |
7.0 |
6 |
93 ± 3 |
120 |
2.25 · 10–3 |
|
1.2 |
KHSO4 |
31 |
9.26–7.20 |
7.0 |
111 |
83 ± 3 |
2520 |
1.48 · 10–4 |
|
1.3 |
KHSO4 |
30 |
8.25–7.86 |
4.2 |
415 |
50 ± 6 |
360 |
1.11 · 10–4 |
|
1.4 |
KHSO4 |
50 |
8.17–7.47 |
5.0 |
113 |
66 ± 4 |
360 |
1.95 · 10–4 |
|
1.5 |
KH2PO4 |
46 |
7.86–7.47 |
2.9 |
57 |
100 ± 8 |
240 |
1.92 · 10–4 |
|
1.6 |
KH2PO4 |
29 |
8.30–7.56 |
3.7 |
181 |
69 ± 6 |
300 |
6.10 · 10–5 |
|
1.7 |
KH2PO4 |
47 |
8.20–7.81 |
2.9 |
60 |
100 ± 8 |
240 |
1.78 · 10–4 |
3. Results
The experiments on the membrane contactor confirmed the most efficient mass transfer when the pH of wastewater was above 12. This experiment was crucial for studying mass transfer kinetics, but adjusting the pH to such a high level isn’t suitable for the ecology and economy of the overall process. An important finding was the better separation efficiency when using KH₂PO₄ compared to KHSO₄, which is why further experiments will continue with KH₂PO₄. Experiments conducted at an increased temperature of 50 °C achieved 100% efficiency. However, due to the increased volume of the absorption solution, even at 50 °C, the mass transfer process included membrane distillation. Water distillation is undesirable in the process of ammonia nitrogen separation from digestate, making 50 °C the upper temperature limit. Another issue was the presence of large particles in the digestate, which caused clogging, cutting, and fouling of the membrane fibres. For this reason, a filtration stage is necessary before the digestate enters the contactor. Most important experiments on semi-operational membrane contactor are listed in Table 1. The values of mass transfer coefficients were higher in experiments with adjusted pH and temperature. However, significant efficiency (69 ± 6%) and mass transfer were also observed in experiment 1.6, where no conditions were adjusted and the model solution closely simulated digestate.
Identifying the ideal combination of pH and temperature was crucial for optimizing the stripping technology. This study explores three temperatures (50, 60, 70 °C) and three pH levels (8, 9, and 10.5). An additional experiment at a pH of 11.5 confirmed that increasing the pH beyond 10.5 had no further effect on efficiency. According to Henry's Law, the experiments conducted at 70 °C achieved the best efficiencies and the highest mass transfer coefficients, but this temperature also led to increased water vaporization. In contrast, experiments at 50 °C showed negligible vaporization, though they were unable to reach even 50% efficiency. The highest efficiency at 50 °C was 42.6% after 45 minutes, which is lower compared to similar experiment with the membrane contactor (Experiments 3,5 and 7 in Table 1). Additionally, extending the reaction time did not improve the results, as steady state was reached in approximately 50 minutes. A pH of 8 also resulted in low efficiency, making it unsuitable for full-scale operation. At this pH, the highest efficiency was 30.2% after 50 minutes (Experiment 2.8 in Table 2), and the process had nearly reached steady state.
In the experiments listed in Table 2, an airflow rate of 120 L/min was used. It was confirmed that reducing the airflow rate to 60 L/min also decreased the efficiency of ammonia desorption within the same time period. After 50 minutes of reaction time, 12.3% of the total ammonia nitrogen was separated at 60 °C and pH 8, while 20.9% was separated under the same temperature and pH 9.
Table 2 : The stripping device experiments
|
Experiment |
Absorbent |
T |
pH |
m0 |
max. efficiency |
t |
Kov |
|
[°C] |
[-] |
[g] |
[%] |
[min] |
[s–1] |
||
|
2.1 |
KH2PO4 |
50 |
8.0 |
36.5 |
14.2 |
40 |
2.61 · 10–4 |
|
2.2 |
KH2PO4 |
50 |
9.0 |
34.5 |
28.2 |
45 |
1.77 · 10–4 |
|
2.3 |
KH2PO4 |
50 |
10.5 |
36.1 |
42.6 |
40 |
2.07 · 10–4 |
|
2.4 |
KH2PO4 |
60 |
8.0 |
38.1 |
23.5 |
50 |
3.21 · 10–4 |
|
2.5 |
KH2PO4 |
60 |
9.0 |
33.7 |
64.1 |
50 |
6.54 · 10–4 |
|
2.6 |
KH2PO4 |
60 |
10.5 |
33.3 |
85.1 |
50 |
8.54 · 10–4 |
|
2.7 |
KH2PO4 |
60 |
11.5 |
35.8 |
75.6 |
50 |
9.77 · 10–4 |
|
2.8 |
KH2PO4 |
70 |
8.0 |
36.1 |
30.2 |
50 |
9.55 · 10–4 |
|
2.9 |
KH2PO4 |
70 |
9.0 |
36.0 |
75.9 |
50 |
1.09 · 10–3 |
|
2.10 |
KH2PO4 |
70 |
10.5 |
37.9 |
78.9 |
50 |
1.51 · 10–3 |
The results indicate that the optimal conditions for this semi-operational stripping device are a temperature of 60 °C and a pH range of 9–10.5. In comparison, the membrane contactor appears to be more efficient for ammonia separation than the stripping device, but it requires precise conditions. Conversely, the stripping process is preferable for coarse dispersions without prior filtration. For this reason, it would be beneficial to develop a technology that combines the strengths of both stripping and membrane processes.
4. Conclusion
This study provides a comparative analysis of two semi-operational technologies for ammonia removal from high-ammonia wastewater: the membrane contactor and the stripping device. The results demonstrated that the membrane contactor, using KH₂PO₄ as the absorbent, achieved a maximum ammonia separation efficiency of 100% at elevated temperatures, with 69 ± 6% efficiency reached without any adjustments to operating conditions. In contrast, the stripping device achieved its best results at a pH of 10.5 and a temperature of 60°C, with 85.1% efficiency within 50 minutes. However, the membrane contactor required precise control of parameters such as pH and temperature, while the stripping device proved more adaptable to coarser dispersions without pre-filtration but showed significant dependency on airflow rates and pH levels.
The accuracy of the experimental results may be influenced by the specific limitations of both tested technologies. In the stripping device, temperature fluctuations due to unavoidable heat loss represent a potential source of error, as the system cannot be fully insulated. This variability may slightly affect ammonia removal efficiency. In the membrane contactor, the small inner diameter of the hollow fiber membranes (228 µm) increases the risk of clogging from fine particles in the solution. When clogging occurs, only a portion of the fibers remains passable, reducing the effective interphase mass transfer area and potentially leading to lower-than-expected absorption efficiency. While continuous monitoring and maintenance were implemented to mitigate these effects, such factors introduce variability that must be considered when interpreting the results. Future research should explore improved insulation methods for the stripping device and enhanced pre-filtration strategies to prevent membrane clogging, ensuring more consistent and accurate performance of both technologies.
Each technology presents unique advantages and limitations. The membrane contactor is more suitable for applications requiring fine-tuned operating conditions, whereas the stripping device is preferable for faster ammonia removal under less controlled conditions. Future research should focus on integrating these two approaches to maximize ammonia recovery, combining the strengths of both technologies to enhance efficiency while reducing operational costs. Such advancements could significantly contribute to improving the sustainability of ammonia management in biogas plants.
Acknowledgements
This work was supported by the project "Hollow Fiber Heat Exchangers with Reduced Permeability for Smart Cities", funded as project No. 8I24002 by Programme EIG CONCERT by Ministry of Education, Youth and Sports. The contribution was also supported by the project EPSILON 4 No. FW06010715, Elimination of volatile substances from wastewater with simultaneous conversion into a secondary usable raw material using microporous hollow fibers, granted by the Technology Agency of the Czech Republic.
References
[1] E. Buijsman, H. F. M. Maas, and W. A. H. Asman, "Anthropogenic NH3 emissions in europe", Atmospheric Environment, vol. 21, no. 5, pp. 1009-1022, 1987. View Article
[2] T. H. Misselbrook, "Ammonia emission factors for UK agriculture", Atmospheric Environment, vol. 34, no. 6, pp. 871-880, 2000. View Article
[3] "Re-use of digestate and recovery techniques", in Trace Elements in Anaerobic Biotechnologies, IWA Publishing, 2019, pp. 181-213. View Article
[4] S. Kumar, Biogas. Croatia: InTech, 2012. View Article
[5] S. T. Nesaratnam, Ed., Water pollution control. Wiley, 2014. View Article
[6] B. Teichgräber and A. Stein, "Nitrogen elimination from sludge treatment reject water - comparison of the steam-stripping and denitrification processes", Water Science and Technology, vol. 30, no. 6, pp. 41-51, Sep. 1994. View Article
[7] D. W. Green and M. Z. Southard, Perry's chemical engineers' handbook, Ninth edition, 85th anniversary edition. New York: McGraw Hill Education, [2019].
[8] G. Provolo, F. Perazzolo, G. Mattachini, A. Finzi, E. Naldi, and E. Riva, "Nitrogen removal from digested slurries using a simplified ammonia stripping technique", Waste Management, vol. 69, pp. 154-161, 2017. View Article
[9] A. Serna-Maza, S. Heaven, and C. J. Banks, "Biogas stripping of ammonia from fresh digestate from a food waste digester", Bioresource Technology, vol. 190, pp. 66-75, 2015. View Article
[10] J. Bousek, D. Scroccaro, J. Sima, N. Weissenbacher, and W. Fuchs, "Influence of the gas composition on the efficiency of ammonia stripping of biogas digestate", Bioresource Technology, vol. 203, pp. 259-266, 2016. View Article
[11] L. Kinidi, I. A. W. Tan, N. B. Abdul Wahab, K. F. B. Tamrin, C. N. Hipolito, and S. F. Salleh, "Recent Development in Ammonia Stripping Process for Industrial Wastewater Treatment", International Journal of Chemical Engineering, vol. 2018, pp. 1-14, Jul. 2018. View Article
[12] J. Xiong, Z. Zheng, X. Yang, X. Dai, T. Zhou, J. He, and X. Luo, "Recovery of NH3-N from mature leachate via negative pressure steam-stripping pretreatment and its benefits on MBR systems: A pilot scale study", Journal of Cleaner Production, vol. 203, pp. 918-925, 2018. View Article
[13] A. T. Ukwuani and W. Tao, "Developing a vacuum thermal stripping - acid absorption process for ammonia recovery from anaerobic digester effluent", Water Research, vol. 106, pp. 108-115, 2016. View Article
[14] L. Cao, J. Wang, T. Zhou, Z. Li, S. Xiang, F. Xu, R. Ruan, and Y. Liu, "Evaluation of ammonia recovery from swine wastewater via a innovative spraying technology", Bioresource Technology, vol. 272, pp. 235-240, 2019. View Article
[15] M. Darestani, V. Haigh, S. J. Couperthwaite, G. J. Millar, and L. D. Nghiem, "Hollow fibre membrane contactors for ammonia recovery: Current status and future developments", Journal of Environmental Chemical Engineering, vol. 5, no. 2, pp. 1349-1359, 2017. View Article
[16] A. Hasanoğlu, J. Romero, B. Pérez, and A. Plaza, "Ammonia removal from wastewater streams through membrane contactors: Experimental and theoretical analysis of operation parameters and configuration", Chemical Engineering Journal, vol. 160, no. 2, pp. 530-537, 2010. View Article
[17] D. Yang, Q. Chen, R. Liu, L. Song, Y. Zhang, and X. Dai, "Ammonia recovery from anaerobic digestate: State of the art, challenges and prospects", Bioresource Technology, vol. 363, 2022. View Article
[18] Y. Li, X. Hu, Z. Wu, and Y. Sun, "Review of liquid-liquid hollow fiber membrane contactor for ammonia recovery from wastewater: Membrane, feed and receiving solution", Journal of Environmental Chemical Engineering, vol. 12, no. 5, 2024. View Article
[19] J. Zhang, M. Xie, X. Tong, S. Liu, D. Qu, and S. Xiao, "Recovery of ammonium nitrogen from human urine by an open-loop hollow fiber membrane contactor", Separation and Purification Technology, vol. 239, 2020. View Article
[20] Y. Huang, C. Xiao, Q. Huang, H. Liu, and J. Zhao, "Progress on polymeric hollow fiber membrane preparation technique from the perspective of green and sustainable development", Chemical Engineering Journal, vol. 403, 2021. View Article
[21] F. Rivera, R. Muñoz, P. Prádanos, A. Hernández, and L. Palacio, "A Systematic Study of Ammonia Recovery from Anaerobic Digestate Using Membrane-Based Separation", Membranes, vol. 12, no. 1, 2022. View Article
[22] B. Riaño, B. Molinuevo-Salces, M. B. Vanotti, and M. C. García-González, "Ammonia Recovery from Digestate Using Gas-Permeable Membranes: A Pilot-Scale Study", Environments, vol. 8, no. 12, 2021. View Article
[23] F. Wäeger-Baumann and W. Fuchs, "The Application of Membrane Contactors for the Removal of Ammonium from Anaerobic Digester Effluent", Separation Science and Technology, vol. 47, no. 10, pp. 1436-1442, 2012. View Article

