Volume 12 - Year 2025 - Pages 195-206
DOI: 10.11159/jffhmt.2025.021
Enhancing Methane Yield via Biomethanation and Kinetic Modelling
Clement Dabanga, Zahir Dehouchea, Jan Wissinka, Adejumoke Adeotib, ,Gideon Baklitc, Samuel Emebud , Guy Blanche, Rokia Yamane
aCollege of Engineering, Design and Physical Sciences, Brunel University London, Uxbridge, UB8 3PH, UK
a*Brunel Business School Brunel University London, UB8 3PH, UK
bDepartment of Geography, Faculty of Environmental Science, University of Jos, Nigeria.
c Department of Separation Science, Lappeenranta-Lahti University of Technology LUT, Yliopistonkatu 34, FI-53850 Lappeenranta, Finland
dLEAP micro AD,193 Downham Way, London, BR1 5EL UK
First:Email: clement.dabang@brunel.ac.uk; Second:Zahir.Dehouche@brunel.ac.uk
Third.Jan.Wissink@brunel.ac.uk
Abstract - This investigation explores the enhancement of CH4 generation in anaerobic digesters (AD) via in-situ renewable hydrogen injection utilising four exotic crop wastes and a crop (five feedstocks). The substrates are yam, cassava, and cocoyam peel (YP, CP and CYP), rice husk (RH) and finger millet seeds (FMS). Biomethane Potential (BMP) Tests, followed by AD experiments with food waste inoculum (FWI), were conducted in triplicate under mesophilic conditions (37°C), utilising an anaerobic model (ANM) test rig. The last phase of the experimental campaign is bio-methanation to upgrade CH4 purity. CYP and YP showed 233% and 81.5% higher gas yields, respectively, with CH4 content improvements up to 38.5%. However, CP emerged as the optimal feedstock, hence the primary substrate utilised in the AD, supporting hydrogenotrophic methanogenesis (HM) and CO₂ to CH₄ conversion. Consequently, MATLAB-based kinetic modelling confirmed the Richard equation as the best fit predictor. The novelty of this study lies in the innovative incorporation of in-situ H₂ injection (0.67 ml/min), bubble mixing and mass transfer to enhance CH₄ from tropical crop waste (cassava peel), a widely available yet underutilised feedstock specific to Plateau State, Nigeria. Additionally, integrating computational fluid dynamics (CFD) and bioprocess kinetic modelling provides a comprehensive framework for understanding the parameterisation and optimising system dynamics. This consolidates the research contribution to the experimental optimisation of decentralised biogas systems, facilitating sustainable energy solutions for pipeline quality in tropical regions.
Keywords: Feedstock BMP characterisation, in-situ renewable hydrogen injection, transport phenomena, kinetic modelling and Plateau State, Nigeria.
© Copyright 2025 Authors - This is an Open Access article published under the Creative Commons Attribution License terms Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2025-01-16
Date Revised: 2025-05-13
Date Accepted: 2025-06-02
Date Published: 2025-06-09
1. Introduction
The rising worldwide energy requirement and growing waste generation due to massive population growth have necessitated extensive research in renewable and sustainable energy systems. This energy system has become imperative as a solution to the rising energy demand. AD seems to be a veritable pathway, considering the overexploitation of fossil fuels and accelerated energy demand. Both are responsible for the substantial decrease in fossil fuel abundance in the Earth’s natural reserves [1]. Unlike fossil fuels, biomass energy conversion requires limited technical, cost, accessibility demands and environmentally friendly [2]. The bioenergy can be utilised for electricity/heating and refined to meet pipeline-quality fuel standards [3][4].
In developing nations such as Nigeria, where agricultural wastes are plentiful, crop processing waste, decayed crops due to insufficient storage facilities, disease-infected crops, and crops affected by pest infestations are also included [5]. AD holds significant potential for decentralised energy generation [6]. However, CH4 yield, and purity limitations often restrict biogas systems from achieving full energy recovery and integration into established energy networks. The composition of biogas comprises CH4 and CO2, ranging from 55 to 65% and 45 to 35%, respectively, along with trace gases such as hydrogen sulphide (H2S), constituting between 0.1% and 3% and ammonia (NH3) [1]. One of the primary issues in this fraction is the incomplete conversion of CO2. Recent investigations have shown the potential H2 assisted pathway to augment CH4 production. This method uses H2 to directly convert CO2 into CH4 within the digester, providing a cost-effective solution for biogas enhancement while preserving anaerobic stability. Similarly, challenges like poor gas-liquid mass transfer and mixing dynamics hold back the scalability of this process [7].
As a result, this investigation explores five commonly consumed exotic crops peculiar to Plateau State, Nigeria. These feedstocks include CP, CYP, YP, RH, and FMS, which were selected based on availability, composition, and relevance to local consumption patterns. The study applies a three-phase experimental campaign, combining BMP testing, standard AD, and bio-methanation. Consequently, a parametric comparative kinetic modelling and a fluid–thermal analysis framework were followed to examine gas mixing, thermal control, and mass transport. The novelty lies in coupling kinetic analysis with momentum, heat, and mass transport assessments to understand their parameterisation and optimise system dynamics. Bridging the gap by integrating experimental findings with MATLAB-based modelling and transport phenomenon analysis. Fundamentally, to develop a practical, scalable framework for improving CH4 yield and biogas purity. The results analysis will help to facilitate sustainable energy policies, waste valorisation methods, and decentralised bioenergy planning across government, academic, and industry sectors.
2. Related Work
2.1. Feedstock Suitability and Energy Recovery from Crop Waste
Nigeria’s agricultural sector produces many exotic crop residues that remain largely untapped for renewable energy recovery. These wastes are readily available for energy generation sources via combustion, pyrolysis and AD [8] [6]. Nigeria is typically a foremost global producer of tuber crops, including cassava (approximately ~ 63 Mt/yr.)[9] [10] cocoyam (~2.7 Mt/yr.), yam (~45 Mt/yr.)[11], as well as cereals; rice (82 Mt/yr.) [12] and finger millet (1.5 Mt/yr.)[13]. These feedstocks have differing biochemical characteristics, which influence their biodegradability, energy yield, and process kinetics during AD. The abundance of these high-energy crops, with typical Low Heating Value (LHV) of 16.4, 16.43, 16.43, 16.4, and 15.4 MJ/kg for cassava, cocoyam, yam, rice and finger millet, respectively [14], making their respective generated waste a potential source of bioenergy.
Typically amount of waste, i.e. cassava (~37.8 Mt/yr.), cocoyam (~1.7 Mt/yr.), yam (~2.9 Mt/yr.) peels, rice husk (~2.6 Mt/yr.) and finger millet straw (2.2 Mt/yr.) are produced with a corresponding energy content of 10.61, 14.24, 16.4, 16.02 and 15.4 MJ/kg [15] [6]. Oguntoke et al. earlier reported that 82 Mt/yr of crop waste, with a biogas estimate of ~4.98 billion m3/yr, can be used to generate 117,000 TJ/yr of energy[5].
2.2 Lignocellulosic Feedstock Limitations and Energy Recovery
Lignocellulosic biomass, like RH and FMS, presents a very high energy potential, limited by poor hydrolysis and high fibre content, which limits degradability. The biochemical resistance of the Lignocellulosic Fraction poses a significant hurdle in AD [16]. This result aligns with the current findings, where feedstocks like RH showed delayed and reduced methane output, necessitating pre-treatment or co-digestion strategies for enhanced performance [17], [18], [19]. CP valorisation showed its potential as a value-added product source beyond simple waste treatment [19].
2.3. In-Situ Hydrogen Injection and Hydrogenotrophic Methanogenesis (HM)
Bio-methanation in the upgradation of methane in anaerobic systems offers a compelling route by consuming CO₂ to CH4 via HM and AM (Acetoplastic Methanogenesis)[1]. It has also been reported that enriching hydrogenotrophic methanogens like Methanothermobacter, can utilise H₂ and CO₂ directly to boost CH₄ purity [21], [22]. The prevalence of AM (Methanosarcina) and HM (Methanobacterium) species is contingent upon the substrate utilised in AD. Regarding thermodynamic stability, the HM pathway is more advantageous than the AM pathway [1]. Their stoichiometry HM converts 1 mole of CO₂ and 4 moles of H₂ into 1 mole of CH₄, as expounded by Lai et al. [21]. The AD HM route accounts for up to 30% of the CH4 content in biogas composition, with reduced H2 concentration [1].
Recent studies show bio-methanation significantly improves CH₄ content by stimulating HM [20], [26]. It was also demonstrated that recirculating gases and supplying H₂ in methanogenic reactors enhance CO₂-to-CH₄ conversion[21]. However, H₂ mass transfer limitations and microbial inhibition at high partial pressures remain a significant challenge [26]. Some in-situ biogas upgradation techniques were also reviewed in another study, highlighting the need for optimised H₂ dispersion to prevent energy losses [23]
2.4. Role of Mass and Momentum Transfer
The crux and the innovation in this investigation is the integration of bubble-induced mixing, microbial uptake efficiency, and thermal dynamics within the AD systems on exotic crop waste peculiar to Plateau State, Nigeria. Computational Fluid Dynamics (CFD) based modelling was used to buttress bubble behaviour and interfacial area optimisation to boost H2 solubility and microbial accessibility [1], [24]. Properly comprehending bubble dynamics, mixing substrate, and microorganism interactions is key to optimising reactor performance. This is a state-of-the-art review of gas absorption using CFD modelling to explain vividly how the transport process governs conversion efficiency. In anaerobic systems, poor hydrogen dispersion can limit microbial access, reducing methane production efficiency, as observed in this experiment.
Understanding this process is necessary, considering the mass transfer mechanism and dynamism in moist biomass systems with peels and pulp-based feedstocks [23]. This investigation also confirms how biogas affects mixing efficiency. Therefore, overcoming mass transfer barriers using microbubble diffusion techniques can further inform and improve future reactor design refinements for this kind of system, as observed in this experiment[23].
2.5. Kinetic Modelling for Predictive Optimisation
As the best-fitting kinetic expression, Richard's model demonstrates an advanced approach to modelling dynamic typical digestion behaviour. This expression aligns with the growing body of literature supporting non-linear models like Gompertz, Richards over simple first-order kinetics, especially when accounting for lag phases, multi-phase transitions and microbial adaptation [23], [25]. The MATLAB-based kinetic simulation in this investigation concretises mass transfer coefficients and microbial growth constant. Integrating explicit physical transport parameters and biological uptake
2.6. Towards Pipeline-Quality Methane and Decentralised Energy
The research capability’s reaching 75% CH4 purity without complete CO2 removal confirms the possibility of AD as a probable energy generation system in Nigeria. Considering the strategic alignment of feedstock selection/characterisation, transport mechanism and microbial targeting is a precise gradation to the possibility towards a local, decentralised biogas solution. The residual CO₂ fundamentally limits energy density, showcasing onsite calorific value upgrading of (~22 MJ/m³) [26]. This innovative study bridges the gap by showcasing a scalable H₂ Injection model under mesophilic conditions.
However, advances in bio-methanation and CFD modelling [21], [7] demonstrate the technical feasibility of producing pipeline-quality CH₄ ≥ 90% from exotic crop waste. Plateau State accounts for over 1.2 million tons/year of cassava and RH waste [16]. The absence of BMP standards in the National Renewable Energy Policy (NREP, 2015) creates a critical adoption barrier. Consequently, the evidence of HM achieving CH₄ purity of ≥ 95% is visible as far as:
- Policy-driven incentives like feed-in tariffs for cleaned biogas are available
- Modular upgrading systems (e.g., membrane separation and pressure swing adsorption for decentralised use are also available [26].
Though the kinetic models optimise CH4 yield performance, the knowledge gap is their scalability contingent on state-level energy planning. Few studies in West Africa have examined how government policies can help decentralised small-scale biogas systems, especially in Plateau State, for high-quality and efficient methane generation.
3. Materials and methods
3.1. Biomethane Potential (BMP) Test
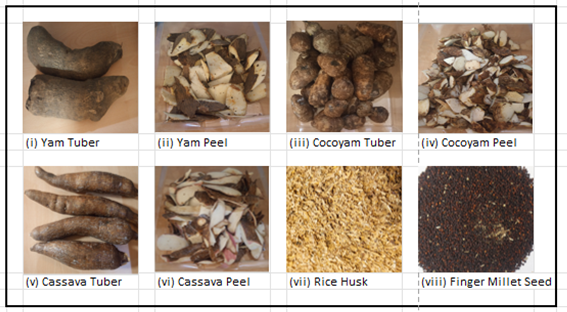
Phase I incorporate BMP testing on four waste-derived samples (YP, CP, CYP, and RH) and one newly discovered crop, finger millet seeds (FMS), sourced from Plateau State, Nigeria. While waste-based feedstocks were analysed, FMS was included to evaluate its potential as an energy crop before considering its waste for future studies. Figure 1 shows three crops and their waste components, RH and FMS. 500 g of all feedstocks were transported to NRM Laboratories (Bracknell) for characterisation. Samples were dried, ground, and characterised for proximate and ultimate analysis. Their respective theoretical bio-methane potential and volatile solids (VS) were also determined. Characterisation was defined using standard methods for analytical chemistry procedures, also known as physicochemical properties (PP).
3.2. Anaerobic Digestion (AD)
Phase II involves inoculum preparation, feedstock sourcing/preparation, and AD experimentation. Food waste inoculum (FWI) was utilised for the experimentation, extracted from a food waste biodigester in the Celignis laboratory, and stored in a 30° C incubator. It was degassed under mesophilic conditions (37 ° C) for a week before utilisation. Feedstocks were injected in wet conditions, and the inoculum was used after 5 days of collection. 3.2 g each was collected from CP, CYP, and YP, 4.45 g for RH and 4.2 g for FMS. FMS was pounded, RH was milled, and the husk was collected (see Figure 1). FMS, RH, CP, CYP, and YP substrates were tested in 1 L (1000 ml) batch digesters known as the Anaero Nautilus Bioreactors (ANB) (see figure 2) at 37°C and 1 atm. The experiment followed the VDI 4630 (2016) standards protocols, with an inoculum-to-substrate ratio (ISR) of 4:1 on a volatile solid (VS) basis. The experiments were conducted in triplicate. ANB consist of fifteen one-litre containers, but only 700ml is actively used, fully immersed in the Nautilus water bath with a tight water cover, reducing evaporation. The system was continuously shaking to maintain and simulate semi-continuous operations. Energy balance calculations were performed to assess the net energy gain, while the theoretical methane yield was benchmarked at 374 L/kg VS.

3.3. In-situ Renewable Hydrogen Injection
Phase III involved bio-methanation. H2 generated by renewably powered electrolysis is forcefully broken down from water molecules through electrolysis, collected, stored, and injected in situ directly during the AD. H2 mass flow rate was continuously injected at 0.67ml/min and introduced through a porous diffuser at a stoichiometry ratio of H2: CO2 = 4:1 (see equation 1). Operating conditions remained 1 atm in mesophilic conditions (37 ° C). HM were enriched to boost metabolic activity. Gas chromatography (GC) analyses of CH4 and CO2 were calibrated while CH4 content, total biogas volume, and pH were monitored daily. Kinetic modelling was done using three models: First-order, modified Gompertz, and Richards. Parameters such as lag phase, maximum methane production rate, and ultimate methane yield were estimated. Mass and heat transfer analysis included bubble dispersion patterns and microbial uptake assumptions, drawing from validated parameters in the literature.
4. Results and Discussion
4.1. Feedstock Characterisation
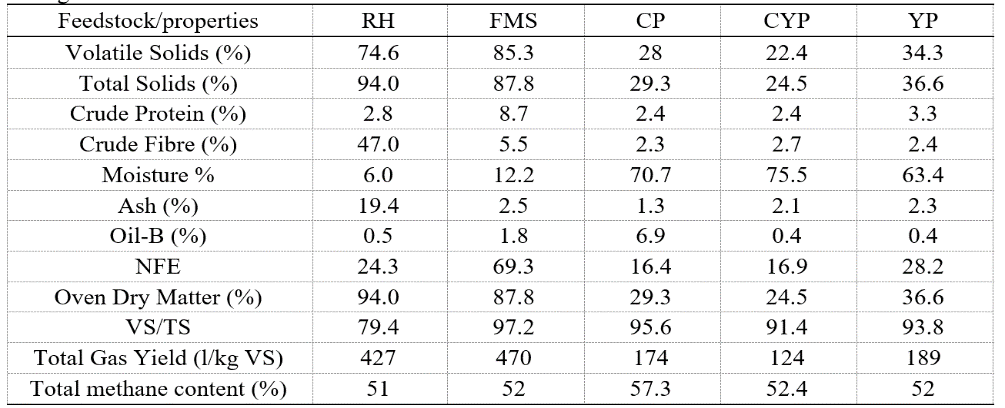
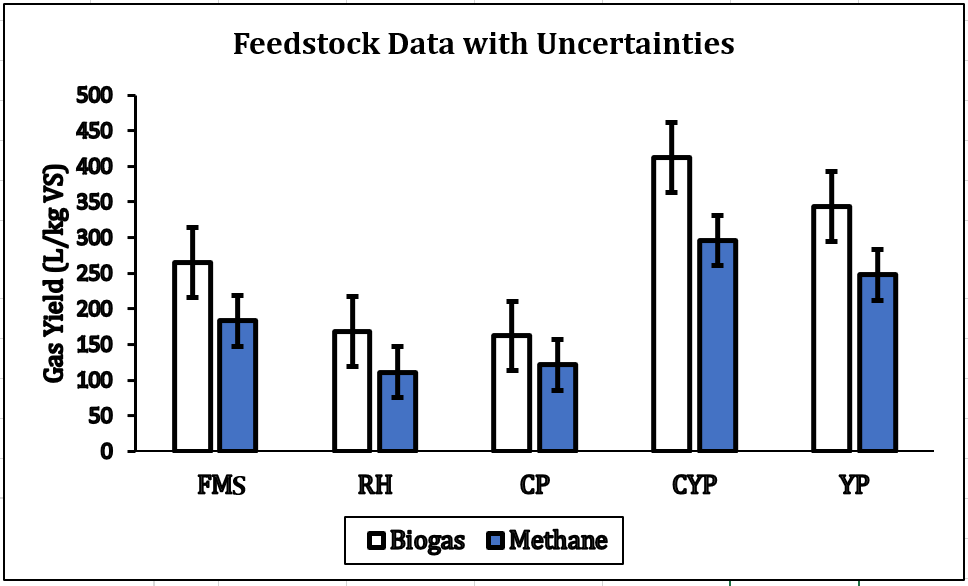
As shown in Table 1, FMS exhibited the highest VS/TS ratio of 97%, and a gas yield of 470 L/kg VS, indicating superior biodegradability. RH shows slower degradation due to a high % fibre content of 47% [27], though it is high in TS with 94%. Moist feedstocks like CP, CYP, and YP may reduce water demands but pose viscosity risks [19]. CP has the highest CH4 purity of 57.3%, making it a strong candidate for energy recovery despite a lower total yield (174 L/kg VS). However, these properties influence mass and heat transfer in degradability [24] based on their significant digestibility and momentum via mixing efficiency during reaction.
4.2. Anaerobic Digestion
Phase two is the AD. The analysis of Table 2 revealed that CP, CYP and YP had substantial CH4 increase from +17.7 % to +20%. CYP showed significant improvement, having 413 L/kg VS (+233% gas yield). However, these properties, influence mass and heat transfer in highlighting the role of pre-treatment in enhancing biodegradability in optimising AD outcomes. CP, YP and CYP had the highest post-AD CH4 content of 75% and 72%, respectively, with CYP achieving a +19.6% increase. FMS and RH had reduced gas yields (−43.6% and −39.3%) but modest CH4 increases of +7% and +4%, indicating less efficient substrate utilisation without pre-treatment. Figure 3 shows a CYP of 413, and a YP of 343 L/kg VS. However, higher CH4 content, especially in CYP and YP, signifies greater energy density by improving thermal efficiency. Considering C/N ratios, maintaining an optimal level of 20:1 and 30:1 is crucial to promoting stable microbial activity, while also preventing ammonia/acid toxicity and enhancing CH4 yield performance[16], [19].
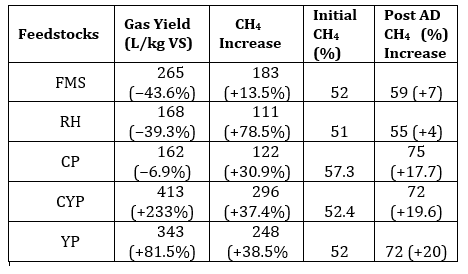
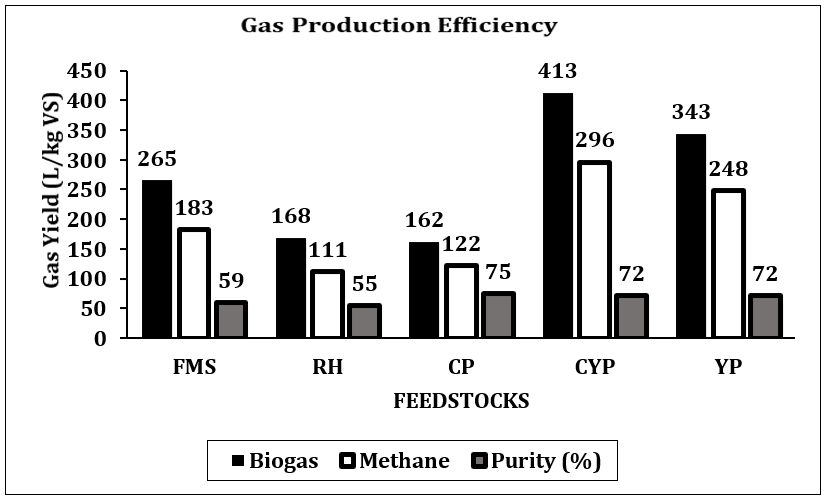
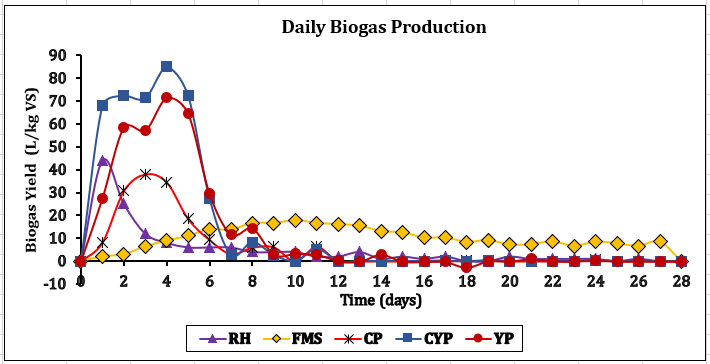
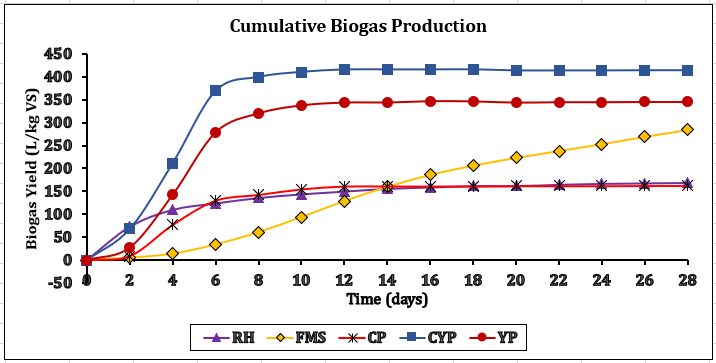
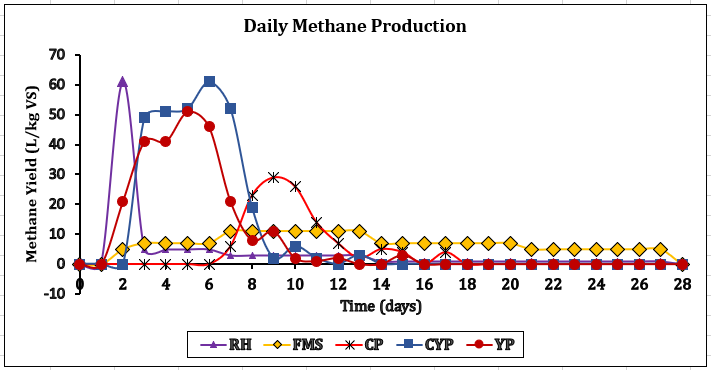
CH4 increase, signifying effective methanogenesis. Consequently, if the C/N is unbalanced, co-digestion or feedstock adjustments are necessary for smooth AD. All tested feedstocks (TBF) achieved >70% CH₄ content, nearing pipeline-grade quality (>80% CH₄), confirming in-situ upgrading potential [26]. CYP and YP achieved the most significant CH₄ enrichment rates at +37.4% and +38.5%. CP achieved a steep increase in CH₄ yield by +30.9% after experiencing a 7-day lag phase because of cyanogenic glycosides. Biogas with increased CH₄ content exhibits approximately 50% more energy density than untreated biogas. Figure 4 demonstrates that CYP achieved the maximum biogas production at 413 ± 27 L/kg VS and the highest biomethane output at 296 ± 19 L/kg VS. This is the best for gas production and the most energy-rich gas production. YP is second, while CP is the most reliable, but the lower-yield FMS has high variability. Figures 5 and 6 showed that FMS achieved the fastest production start on Day 1 but produced the lowest CH₄ upgrade at +13.5%, persisting throughout the entire 28th day with a yield of 265 L/kg VS. Furthermore, Figure 6 shows the corresponding transient biomethane production, except for CP, experienced a 7-day lag period before increasing production until 15 days. CP started on the 8th day. The delay in CP may be because of high amounts of cyanogenic glycosides. The seven-day lag phase led to a quick overall yield due to significant microbial growth during the log phase, which lasted from days 8 to 13. This was followed by minimal biogas production in the death phase on the 14th and 15th days. RH and CP showed moderate yields but distinct kinetics. Key kinetics analysis shows that 80–92% of total biogas peak production occurred between Days 6–19 for most feedstocks. CP's 7-day lag preceded rapid methanogenesis from Days 8 to 15, likely due to microbial adaptation. CYP and YP achieved >95% total production within 15 days, and FMS yield performance from Day 1, with CP's highest purity of 75% CH4 showing process efficiency. However, CYP/YP balanced high yield of >300 L/kg VS) and upgrading potential (+37–38% CH₄) shows both process efficiency and feedstock suitability. However, RH underperformed due to a slower degradation peak on Day 26 and stopped producing, achieving a total volume of 168 L/kg VS. Feedstock selection critically impacts production timelines and CH₄ quality. In-situ hydrogen injection could optimise CP and CYP/YP outcomes. However, CP was chosen for the in-situ bio-methanation because of its highest BMP test value.

4.3. In-situ Renewable Hydrogen Injection
The conversion gradation of AD consists of four stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. Nevertheless, methane production during the four stages can occur through acetoclastic methanogenesis (AM) and hydrogenotrophic methanogenesis (HM). Thus, syntrophic activity between AM and HM must coexist inside the reactor to achieve higher CH4 content in the biogas composition through bio-methanation [1]. Similarly, H2 was introduced into the AD system to consume CO2 to produce more CH4, enhancing methane purity to a Natural Gas Standard of above 90%, the crux of this investigation [28].
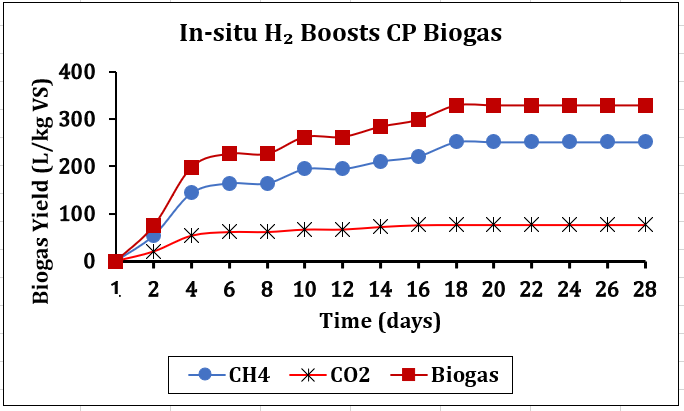
Bio-methanation requires bubble movement within the AD system, essential for facilitating microbial interaction with the substrate. Gas-induced mixing is necessary to increase CH4 levels. Consequently, the significant advancements we observe improve heat exchange, reduce mixing energy, and improve mass transfer in biogas systems. This experiment utilised an advanced test rig, the Anaero Nautilus model (ANM), to optimise technical performance driven by a gearbox for consistent mixing. Recent studies have shown that hydrogen bubbling can boost mass transfer, which in turn helps microbes absorb more by overcoming solubility limits[24], [28]. Figure 7 illustrates a notable rise in CH4 and total biogas, especially between Days 1 and 14, signifying vigorous microbial fermentation. From the 16th to the 28th day, the yield performance steadied, indicating that microbial activity may have attained maximal efficiency [16], [21], [27]. This unique process demonstrated a significant boost in conversion efficiency in reducing CO₂, the impact of H₂, and the evidence of increased CH₄ yield, especially during methanogenesis when the ultimate HM routes were essential. The shift in the biogas composition was very explicit, confirming yield efficiency.
By introducing H₂, the lag phase was significantly reduced, thanks to an earlier surge in microbial activity during digestion [6], [28]. This led to a faster increase in biogas production, while the purity remained steady. Figure 7 illustrates how improved breakdown of lignocellulosic materials in CP resulted in more efficient substrate utilisation and higher overall gas yields. This is crucial for converting complex carbohydrates into simpler molecules that are more responsive to fermentation [29], [30].
Better mixing can improve gas-liquid interaction by creating the conversion gradient of AD. Nevertheless, methane production during the four conversion stages can occur through acetoclastic methanogenesis (AM) and hydrogenotrophic methanogenesis (HM). Thus, syntrophic activity between AM and HM must coexist inside the reactor to achieve a higher CH4 content in the biogas composition.
We can also improve Gas-liquid interaction by creating smaller bubbles to increase biogas efficiency in the systems [7], [22], [23].
The assay's Hydrogen equivalent injection mass flow calculation on the molar ratio stoichiometry in the balanced chemical equation was 0.67 ml/min. Due to the incomplete chemical bonding between H2 and CO2, the quality dropped from 75% to 51.6%, a relative reduction of 31.2% and an absolute drop of 23.4%, according to a volumetric analysis. This task encompasses technological problems such as managing, stabilising processes, key performance metrics and manipulating specific microbial pathways during biogas generation. The good news is that biogas increased from 162 to 329 L/kg of VS and biomethane from 120 to 252 L/kg of VS. Biogas and biomethane increased by 101.8% and 110%. Consequently, even with some CO₂ conversion, we could not reach full methanation due to the reactor's design and microbial population limitations. While bubbles' movement affects how bacteria engage with substrates, we still need a small quantity of extra gas-induced mixing to ramp up CH4 production. This noteworthy improvement implies that we may significantly enhance CH4 quality and raise the efficiency of biogas systems by improving heat exchange and mass transfer while lowering mixing energy [7], [22] [23].
4.4. Comparative Analysis with and without H2
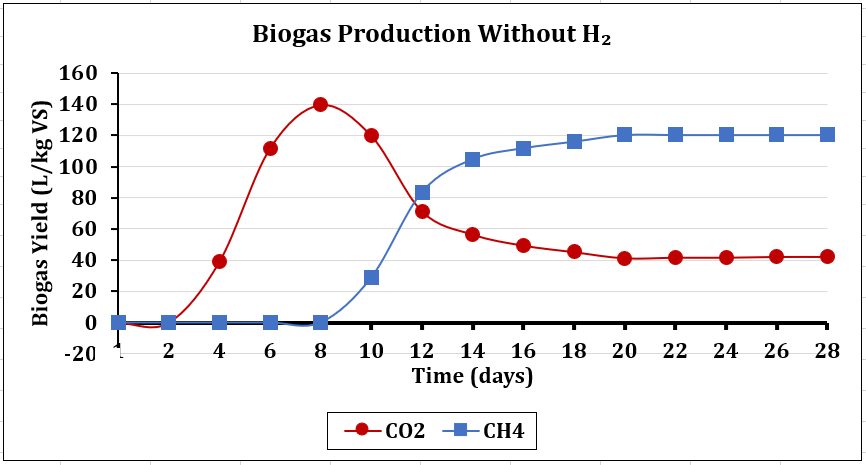
4.4.1. Biogas Composition without H2
Figure 8a showed the AD without H₂ direct input, the CO₂/CH₄ ratio of baseline digestions, as shown with the red line (CO₂) an intriguing trend initially greater than the blue (CH₄), which shifted from the right to the left on the 10th day, gradually falling below the blue line until the 28th day, indicating a predominant acidogenic but low methanogenesis phase. The sharp rise shows a slow hydrolysis reaction, with high CO2 peaking at almost 60% of the total biogas composition during the initial 10 days due to acidogenic methanogenesis. The 7-day lag phase observed impacts overall productivity, with CH4 output beginning to increase only after the seventh day. This suggests that without H2, the system struggled to achieve optimal methanogenic activity. H2 derived from lignocellulosic breakdown [24], [16] is highly responsible for the strong native hydrogenotrophic archaea (Methanothermobacter) [21], [20], impacting high CH4 purity of 75% from the 8th to the 28th day. The rise mirrors fibre-rich feedstock behaviour [16], [20], and the subsequent surge suggests mediated CO2 conversion [26].
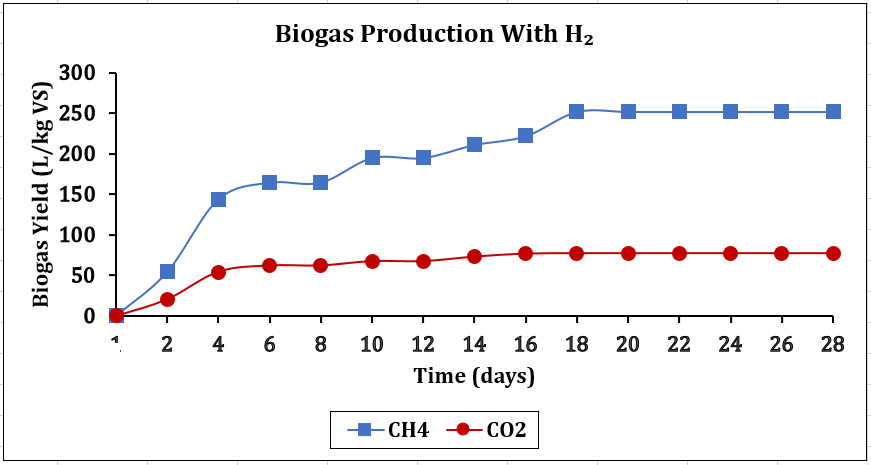
4.4.2. Biogas Composition with H2 Input
Conversely, when H2 was introduced to AD, as shown in Figure 8b, the blue line was above the red line, demonstrating in-situ CO₂ sequestration via HM, offering a promising future research and application outlook. Here, the red line (CO2) consistently remained below the blue line, signifying that the high-powered H2 has humbled the red line, improving biogas dynamics and conversion efficiency [1], [21]. A remarkable increase in microbial activity drove a more effective hydrogenotrophic conversion. The result was a significant increase in biogas density, with the dynamics shifting from a CO2 state to a balanced state until the 28th day.
4.4.3. Mechanism of Improvement
The analytical data reveal significant insights into microbial activity and substrate dynamics of H₂ and CO₂ interplay in CH4 generation. Comparing Figure 8a and 8b, H2 injection reversed the CO₂/CH₄ baseline digestion ratio from Figure 8a, where the red line is above the blue line, to Figure 8b, where the blue line is above the red line, demonstrating in-situ production efficiency. Insights from chemical reactor studies, particularly involving gas absorption towers, are increasingly relevant in optimising AD systems. In this experiment, H2 bubble-induced mixing enhances substrate, CO2 and microbe contact, driving conversion and enhancing methane generation efficiency [7]. However, poor H2 dispersion can also limit microbial access, demonstrating that microbial activity is paramount in this experiment [22]. This aligns with this finding, where microbial activity and reaction rates are affected by self-heating due to microbial metabolism, reinforcing the importance of thermal modelling in kinetic prediction [20]. Integrating CFD techniques enables precise mapping of gas interactions, significantly enhancing the efficiency and accuracy of biogas systems [16]
(a)
(b)
4.5. Models
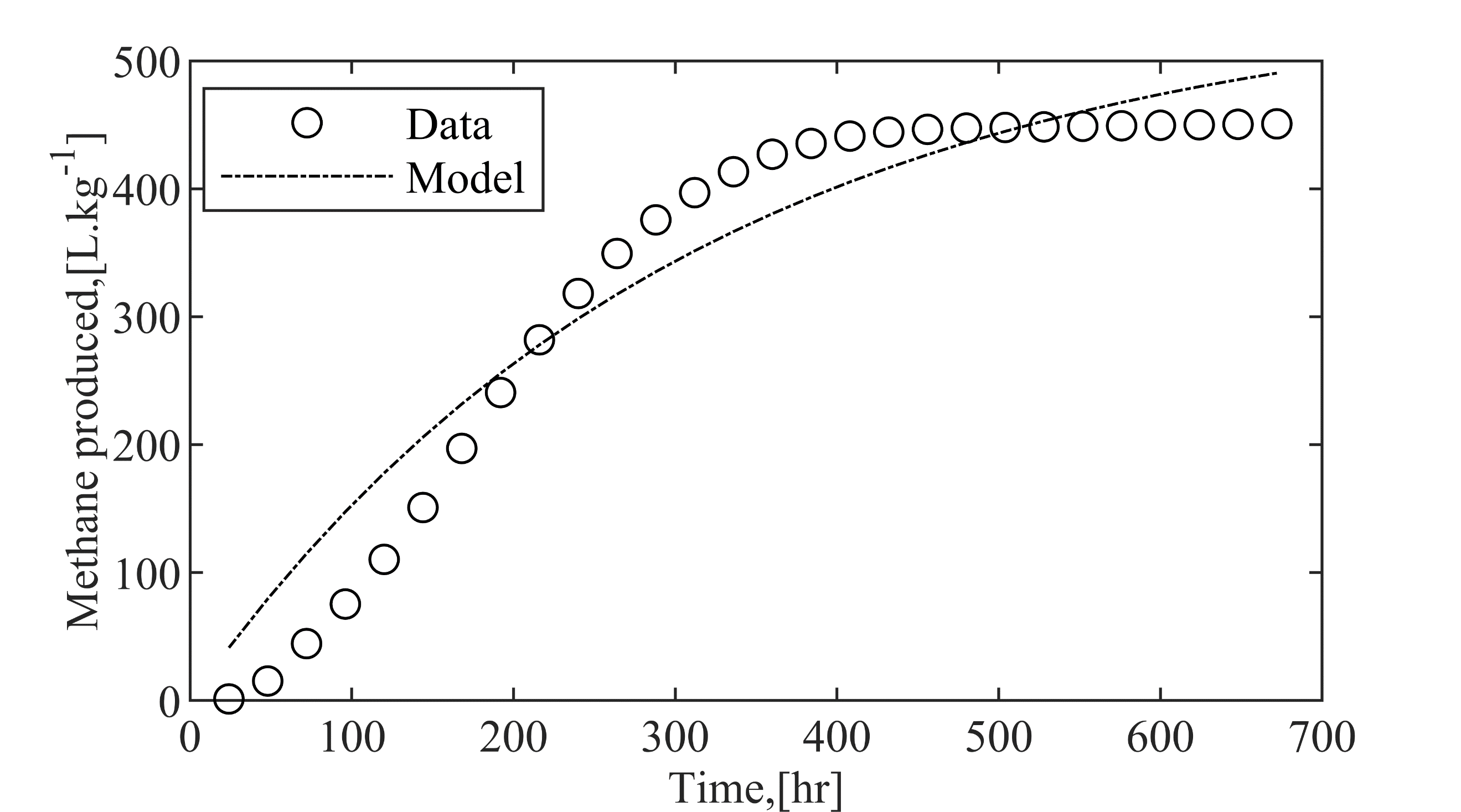
(a) First-Order Kinetic Model

(b) Modified Gompertz Model
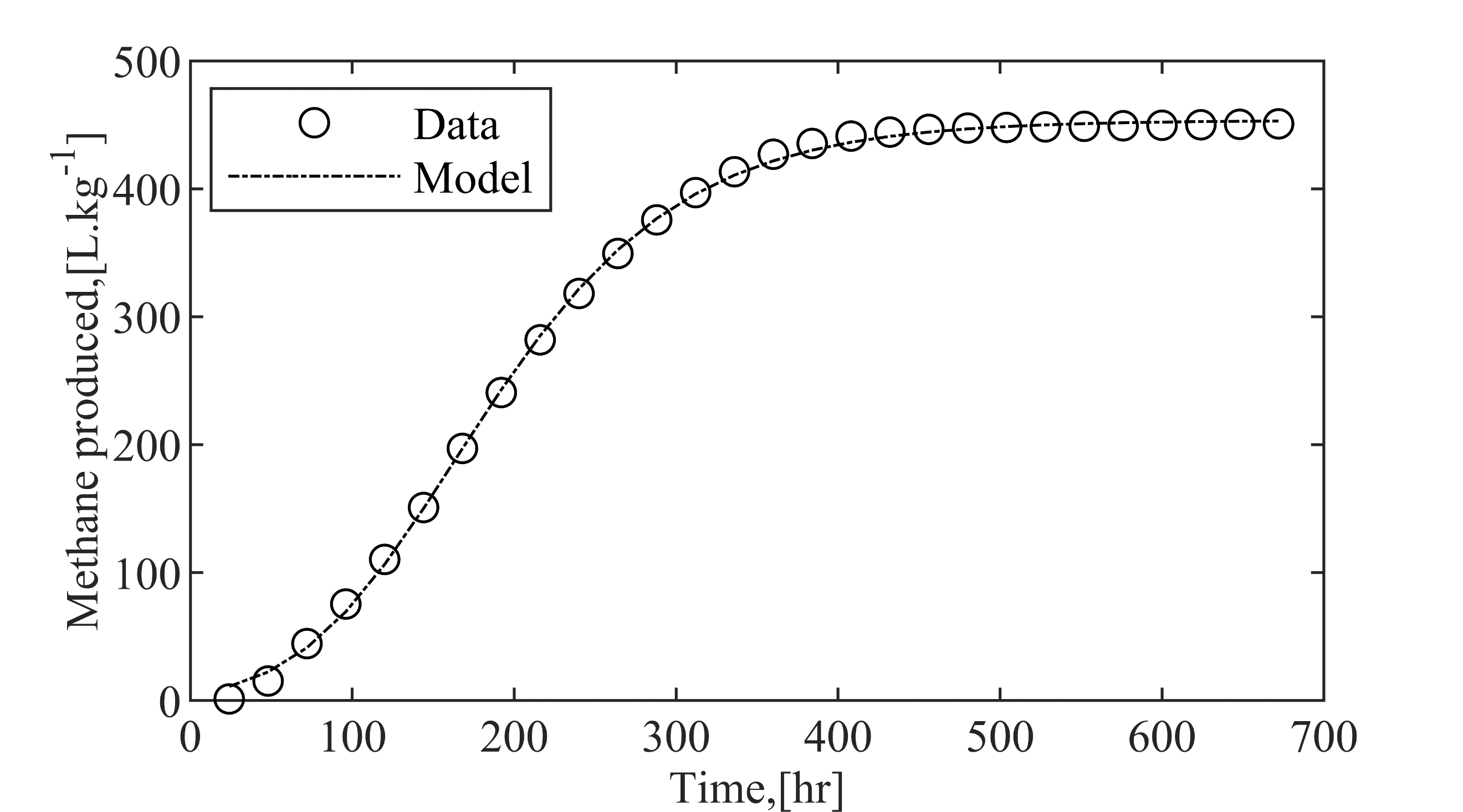
(c) Richard Model
Every model we examined included the lag phase, essentially the time it takes for microbes to adapt to their new environment, particularly at the beginning of anaerobic digestion[31]. Interestingly, the Richard and Modified Gompertz model was the most effective choice for simulating dynamic biogas systems. Energy analysis
showed a net positive energy output, confirming that injecting H2 in mesophilic environments is a promising approach [7]. The partial CH₄ enrichment we achieved aligns with the latest trends of upgrading biogas in the digester instead of doing it downstream.
![]() Equation (2)
Equation (2)
4.5.1 Model evaluation
The accuracy or evaluation metrics for the proposed kinetic models can be checked using R-squared (R2), Equation (2). The R2 value is between 0 and 1, with a higher value indicating a better model fit. Where y is the output, is the mean output of the data set, and is the model output. (L.kg-1) is the biogas yield, (hr-1) is the first-order kinetic constant, (L.kg-1. hr-1) is the maximal biogas production rate, (hr.) is the latency phase, and delta is the curve-fitting shape factor. These models were curve-fitted to the experimental data in MATLAB using the lsqcurvefit function.
These findings directly support local energy policy by demonstrating how a widely available, yet underutilised tropical crop waste derived from commonly consumed crops specific to Plateau State, Nigeria, can be transformed into renewable pipeline-quality methane. The decentralisation of H₂ enhanced AD for community-scale uptake is the sure way. Providing clean energy while reducing over-reliance on fossil fuels, informing policymakers, and empowering local farmers/entrepreneurs about its unique potential and viability. The energy will be integrated into the energy portfolio, fostering sustainable agriculture practices and promoting economic development.
5. Conclusion
This study presents novel mass transfer principles designed to purify biogas from distinct Plateau State, Nigeria feedstocks. It integrates kinetic models with energy optimisation methodologies to develop cost-effective and scalable anaerobic digestion systems compatible with Nigeria's energy framework.
This innovative approach significantly advances AD, aiming for biogas compositions comparable to pipeline-quality natural gas. Due to optimised fluid dynamics showed a 101.8% increase in biogas yield and a 110% increase in biomethane yield, alongside a 31.2% reduction in purity. The Richard Model (R² = 0.99) validated system performance, highlighting scalability for Nigeria's decentralised energy needs.
Despite incomplete CO₂ transformation due to mass transfer bottlenecks and microbial adaptation, the study highlighted the intricacies of in-situ biogas upgrading engineering. It created awareness of the need to concretise all parameterisations of process dynamics and microbial population management to ensure overall process stability.
While this study demonstrates the potential of in-situ H₂ injection to enhance CH₄ yield from tropical crop waste, certain limitations are acknowledged. First, lab-scale conditions (e.g., controlled mesophilic temperature, homogeneous feedstock) may not fully replicate real-world variability in decentralised systems. Second, the bioprocess kinetic modelling, though robust, assumes ideal microbial consortia behaviour without long-term adaptation effects. Finally, economic and logistical scalability, such as H₂ sourcing in rural Nigeria or digester maintenance, requires further pilot-scale validation. These gaps present opportunities for future work in field trials and techno-economic analysis. Based on the findings, we recommend:
- Subsidies and training programs for local farmers (with small-scale digesters) using tropical crop waste, aligned with Nigeria's National Renewable Energy Action Plan.
- Suggest collaboration and engagement with local government and community organisations to pilot biogas projects based on findings, reducing waste and supplementing LPG use
- This initiative will reduce overreliance on dirty fuels (cow dung, charcoal, and firewood), open up capabilities for rural areas, and, in the long run, generate employment. It will also invariably address energy poverty, waste management, and climate objectives in Nigeria's pursuit of carbon neutrality by 2060.
Future work should focus on continuous-flow systems, dynamic mixing strategies, and advanced microbial consortia to achieve higher methane purities and sustainable implementation at scale to achieve >90% CH₄ purities. This experiment is not a formality because it bridges theory and application, offering a practical path for Global South energy solutions.
Acknowledgements
The authors would like to thank Brunel University for the empirical work and the journal paper, LEAP Micro AD, for granting access to their Anaerobic Digester, particularly Rokia Yaman, for her technical expertise and support during the preliminary stages of the research. Celignis Limited and NRM part of Cawood for helping with the experiment. Also, Mrs Mercy Glong Emmanuel is acknowledged for providing the Finger Millet seed and waste used in the experiment. Lastly, I want to thank all the others who helped make the publication successful.
References
[1] T. C. D’ Silva et al., “Enhancing methane production in anaerobic digestion through hydrogen assisted pathways – A state-of-the-art review,” Renewable and Sustainable Energy Reviews, vol. 151, p. 111536, Nov. 2021, doi: 10.1016/J.RSER.2021.111536. View Article
[2] P. Chowdhury, N. A. Mahi, R. Yeassin, N.-U.-R. Chowdhury, and O. Farrok, “Biomass to biofuel: Impacts and mitigation of environmental, health, and socioeconomic challenges,” Energy Conversion and Management: X, vol. 25, p. 100889, Jan. 2025, doi: 10.1016/j.ecmx.2025.100889. View Article
[3] M. M. Uddin and M. M. Wright, “Anaerobic digestion fundamentals, challenges, and technological advances,” Physical Sciences Reviews, vol. 8, no. 9, pp. 2819–2837, Sep. 2023, doi: 10.1515/psr-2021-0068. View Article
[4] A. Dasgupta, “Anaerobic digestion solutions: advancing circular economy goals in emerging economies,” Waste-to-Energy: Sustainable Approaches for Emerging Economies, pp. 47–65, Jan. 2025, doi: 10.1016/B978-0-443-22356-3.00003-8. View Article
[5] O. Oguntoke, B. A. Amaefuna, M. C. Nwosisi, S. A. Oyedepo, and M. O. Oyatogun, “Quantification of biodegradable household solid waste for biogas production and the challenges of waste sorting in Abeokuta Metropolis, Nigeria,” International Journal of Energy and Water Resources 2019 3:3, vol. 3, no. 3, pp. 253–261, Jul. 2019, doi: 10.1007/S42108-019-00033-9. View Article
[6] O. J. Odejobi, O. O. Ajala, and F. N. Osuolale, “Review on potential of using agricultural, municipal solid and industrial wastes as substrates for biogas production in Nigeria,” Jan. 01, 2024, Springer Science and Business Media Deutschland GmbH. doi: 10.1007/s13399-022-02613-y. View Article
[7] D. Liu, Z. Wang, Y. Cai, J. Chen, J. Jin, and B. Zhao, “Computational Fluid Dynamics Based Modeling of Gas Absorption Process: A State-of-the-Art Review,” Ind Eng Chem Res, vol. 63, no. 18, pp. 7959–8002, May 2024, doi: 10.1021/ACS.IECR.3C02941. View Article
[8] C. Gong, A. Singh, P. Singh, and A. Singh, “Anaerobic Digestion of Agri-Food Wastes for Generating Biofuels,” Dec. 01, 2021, Springer. doi: 10.1007/s12088-021-00977-9. View Article
[9] “Global leading cassava producing countries | Statista.” Accessed: May 02, 2025. [Online]. Available: View Article
[10] S. O. Ebewore and R. A. Isiorhovoja, “Knowledge Status and Disease Control Practices of Cassava Farmers in Delta State, Nigeria: Implications for Extension Delivery,” Open Agric, vol. 4, no. 1, pp. 173–186, Jan. 2019, doi: 10.1515/OPAG-2019-0017/MACHINEREADABLECITATION/RIS. View Article
[11] E. E. Bassey, “CONSTRAINTS AND PROSPECTS OF YAM PRODUCTION IN NIGERIA,” European Journal of Physical and Agricultural Sciences, vol. 5, no. 1, 2017, Accessed: May 02, 2025. [Online]. Available: View Article
[12] L. Mwakyusa, S. Dixit, M. Herzog, M. C. Heredia, R. R. Madege, and N. L. Kilasi, “Flood-tolerant rice for enhanced production and livelihood of smallholder farmers of Africa,” Front Sustain Food Syst, vol. 7, p. 1244460, Nov. 2023, doi: 10.3389/FSUFS.2023.1244460. View Article
[13] S. O. Jekayinfa, J. I. Orisaleye, and R. Pecenka, “An Assessment of Potential Resources for Biomass Energy in Nigeria,” Resources, vol. 9, no. 8, p. 92, Aug. 2020, doi: 10.3390/resources9080092. View Article
[14] M. O. Ukoba et al., “Optimal sites for agricultural and forest residues energy conversion plant using geographic information system,” Heliyon, vol. 9, no. 9, p. e19660, Sep. 2023, doi: 10.1016/J.HELIYON.2023.E19660. View Article
[15] V. Bancal and R. C. Ray, “Overview of Food Loss and Waste in Fruits and Vegetables: From Issue to Resources,” Fruits and Vegetable Wastes: Valorization to Bioproducts and Platform Chemicals, pp. 3–29, Jan. 2022, doi: 10.1007/978-981-16-9527-8_1. View Article
[16] S. Ali, T. A. Shah, A. Afzal, and R. Tabassum, “Exploring lignocellulosic biomass for bio-methane potential by anaerobic digestion and its economic feasibility,” Energy and Environment, vol. 29, no. 5, pp. 742–751, Aug. 2018, doi: 10.1177/0958305X18759009 View Article
[17] A. D. Olugbemide and B. Likozar, “Assessment of Liquid and Solid Digestates from Anaerobic Digestion of Rice Husk as Potential Biofertilizer and Nutrient Source for Microalgae Cultivation,” Processes, vol. 10, no. 5, p. 1007, May 2022, doi.org/10.3390/pr10051007 View Article
[18] A. Reddy, S. Begum, and G. R. Anupoju, “Comparative evaluation of high solids anaerobic digestion of rice husk and rice straw: Impact of substrate characteristics and solids concentration on biogas yield and digestate quality,” Biofuels, 2024, doi: 10.1080/17597269.2024.2384274 View Article
[19] A. M. Nizzy and S. Kannan, “A review on the conversion of cassava wastes into value-added products towards a sustainable environment,” Environmental Science and Pollution Research 2022 29:46, vol. 29, no. 46, pp. 69223–69240, Aug. 2022, doi: 10.1007/S11356-022-22500-3. View Article
[20] “Biogas production potential of co-digested food waste and water hyacinth common to the Niger Delta: Biofuels: Vol 11, No 3 - Get Access.” Accessed: May 13, 2025. doi.org/10.1080/17597269.2017.1358950. View Article
[21] A. Khan et al., “Enrichment of the hydrogenotrophic methanogens for, in-situ biogas up-gradation by recirculation of gases and supply of hydrogen in methanogenic reactor,” Bioresour Technol, vol. 345, p. 126219, Feb. 2022, doi: 10.1016/j.biortech.2021.126219. View Article
[22] I. Angelidaki et al., “Biogas upgrading and utilization: Current status and perspectives,” Biotechnol Adv, vol. 36, no. 2, pp. 452–466, 2018, doi: 10.1016/j.biotechadv.2018.01.011. View Article
[23] F. Ale Enriquez and B. K. Ahring, “Strategies to overcome mass transfer limitations of hydrogen during anaerobic gaseous fermentations: A comprehensive review,” Bioresour Technol, vol. 377, p. 128948, Jun. 2023, doi: 10.1016/j.biortech.2023.128948. View Article
[24] G. D. Saravacos, “Mass Transfer Properties of Foods,” Engineering Properties of Foods: Third Edition, pp. 327–380, Jan. 2014, doi: 10.1201/9781420028805-12 View Article
[25] N. Q. Ren, L. Zhao, C. Chen, W. Q. Guo, and G. L. Cao, “A review on bioconversion of lignocellulosic biomass to H2: Key challenges and new insights,” Bioresour Technol, vol. 215, pp. 92–99, Sep. 2016, doi: 10.1016/J.BIORTECH.2016.03.124. View Article
[26] “Biomass Energy Profiles - B. A. Stout, Food and Agriculture Organization of the United Nations - Google Books.” Accessed: Apr. 11, 2025. [Online]. Available: View Article
[27] N. Bisht, P. C. Gope, and N. Rani, “Rice husk as a fibre in composites: A review,” J Mech Behav Mater, vol. 29, no. 1, pp. 147–162, Jan. 2020, doi: 10.1515/JMBM-2020-0015. View Article
[28] S. Sarker, J. J. Lamb, D. R. Hjelme, and K. M. Lien, “Overview of recent progress towards in-situ biogas upgradation techniques,” Fuel, vol. 226, pp. 686–697, Aug. 2018, doi: 10.1016/J.FUEL.2018.04.021. View Article
[29] E. Maleki, A. Bokhary, and B. Q. Liao, “A review of anaerobic digestion bio-kinetics,” Rev Environ Sci Biotechnol, vol. 17, no. 4, pp. 691–705, 2018. doi:10.1007/s11157-018-9484-z View Article
[30] S. Yilmaz and H. Selim, “A review on the methods for biomass to energy conversion systems design,” 2013. doi: 10.1016/j.rser.2013.05.015. View Article
[31] S. Emebu, J. Pecha, and D. Janáčová, “Review on anaerobic digestion models: Model classification & elaboration of process phenomena,” Renewable and Sustainable Energy Reviews, vol. 160, May 2022, doi: 10.1016/j.rser.2022.112288. View Article

