Volume 12 - Year 2025 - Pages 73-82
DOI: 10.11159/jffhmt.2025.007
Flame stability of Ammonia-LPG Fuel Blend- An Experimental Study
Arnav Banerjee1, D Shanmugasundaram2, Vasudevan Raghavan1
1Thermodynamics and Combustion Engineering Laboratory, Department of Mechanical Engineering,
Indian Institute of Technology, Madras, Chennai, India-600036
2Departamento de Industrias, Universidad Técnica Federico Santa María,
Av. España 1680, Casilla 110-V, Valparaíso, Chile- 2390123
imarnavb4@gmail.com; sdakshnamurthy@usm.cl; raghavan@iitm.ac.in
Abstract - NH3 as an alternate fuel is gaining huge importance in the recent past owing to the urgency to mitigate global warming caused by conventional hydrocarbon fuels. However, NH3 because of its low laminar flame speed and reactivity when compared to those of conventional hydrocarbon fuels, needs to be blended with conventional fuels for it to be a viable alternative. In this paper, NH3 in various proportions is blended with LPG. Experiments are conducted using a single slot burner of 17.3 mm inner diameter for different combinations of parameters like (i) mixing ratios of NH3 and LPG in fuel mixture, (ii) low power ratings (0.1 to 1.1 kW) and (iii) percentages of partial premixing with air (0% to 30%). Analysis of varying the aforementioned parameters is done using flame photographs and trends in flame length data. This is followed by a stability map for different configurations which divides the flames into different regimes of (A) steady flames, (B) stable flames with oscillating tip, (C) flames with vortex shedding, and (D) unstable flames with lift-off. An increase in partial premixing is observed to have a stabilising effect (making flames transition from the regime of vortex shedding to that with oscillating tips) accompanied by shortening of the average flame length, whereas an increase in power rating has the opposite effect. Stable and steady flames are observed with either lower percentage of partial premixing coupled with higher concentrations of NH3 or higher percentage of partial premixing coupled with lower concentrations of NH3. Additionally, the effect of increasing NH3 volume fractions in the fuel mixture on adiabatic flame temperature, NO, CO and CO2 production is also studied using equilibrium calculations and the results are reported.
Keywords: Ammonia-LPG, Diffusion, Partially Premixed, Stability Maps, Temperature, Emissions.
© Copyright 2025 Authors - This is an Open Access article published under the Creative Commons Attribution License terms Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2024-08-30
Date Revised: 2024-12-24
Date Accepted: 2025-02-25
Date Published: 2025-03-03
1. Introduction
In the recent times, global warming has increased tremendously due to the contribution of greenhouse gases released from hydrocarbon combustion. Several research works focus in the direction of carbon-neutralisation in order to mitigate the release of greenhouse gases. Ammonia (NH3) is considered to be one of the alternative fuels of interest [1] owing to its clean energy nature i.e., zero carbon emissions [2]. Being a zero-carbon fuel and an economic hydrogen carrier, its complete combustion products include only steam and nitrogen. However, NH3 combustion comes with its own set of challenges such as lower burning velocity, flammability limit, calorific value and adiabatic flame temperature when compared to conventional hydrocarbon fuels [3]. Therefore, it is difficult to burn pure NH3 in order to establish stable flames [4]. Additionally, ammonia combustion leads to high NOx emissions due to the presence of N atom [5].
To address the above issues, NH3 is often blended with conventional fuels such as hydrogen and various hydrocarbon fuels like methane, gasoline and dimethyl ether (DME). The type of fuel blends and equivalence ratio plays a significant effect in determining the laminar flame speed of the fuel mixture. NH3 blends including methane [3], syngas [6] and dimethyl ether [7] is found to enhance laminar burning velocity (LBV).
Lee et al. [8] observed the increase in LBVs with increasing hydrogen concentration in the fuel mixture at 0.1 MPa. The effect of adding H2, CO and CH4, on NH3/air mixtures was investigated using a heat flux burner by Han et al. [9]. It was found that the maximum LBV shifts towards slightly richer conditions in comparison to pure ammonia, for up to 40% H2 concentration. Lhuiller et al. [10] found an increment in the flame speed from 7 cm/s (0% H2) to nearly 80 cm/s (60% H2) at 298 K. Another study [11] shows that NH3-H2 blends can have LBVs comparable to that of CH4 as H2 ratio increases from
33.3% to 60%. This was also explained by Li et al. [12] using a numerical model. It was found that H2 caused a reduction in overall chemical activation energy and increased transport effect such as mass and thermal diffusion.
Okafor et al. [13] tested the unstretched LBV of a premixed NH3-CH4 flame in a constant volume chamber for different equivalent ratio ranged from 0.8 to 1.3 and the NH3 content (% volume) varied from 0 to 0.3. The findings demonstrated that as NH3 content and equivalence ratio increases, the relation between flame velocity and flame stretching rate changed from linear to nonlinear. Ku et al. [14] investigated the potential of CH4-NH3 blends in expanding spherical premixed flames and measured Markstein numbers, flame structures, and LBVs. Their findings were in close agreement with those of Okafor et al. [13]. The range of these values was further extended to higher pressures, up to 5 bar, by Shu et al. [15]. They found a strong linear relation between LBV and CH4 volume fraction for NH3-CH4 mixtures with the same equivalence ratio. Experimental and numerical study of Lubrano Lavadera et al. [16] investigated the NH3-CH4 flame using a heat flux burner and a spherical chamber. Results were found to differ from one another, especially at higher temperatures, equivalence ratios, and hydrocarbon concentrations where the burner's values were less than 5 cm/s and the chamber's values were greater. Their investigation revealed that, for all NH3 mass fractions, the SL/SL,0 ratio trended linearly, but the temperature behaved non-linearly. Additionally, Liu et al. [17] used CO2 dilution (25–65%) in the oxidizer to study these patterns under oxygen fuel conditions. Across all equivalence ratio, they revealed that burning velocity increased with increasing CH4 content.
Given its carbon-neutral characteristics and improved combustion performance, dimethyl ether DME) is drawing interest as a possible NH3 enhancer [18]. Cai and Zhao's work [7] assessed the laminar flame speed (SL) of NH3-DME flames using a 1D freely propagating flame model. They found that the addition of DME considerably increased the ammonia laminar flame speed to levels that are comparable to those of hydrocarbon fuels. It is also seen that increasing the amount of oxygenated alternative fuels like ethanol [19, 20] and methanol [20] is essential to raising NH3's reactivity and combustion efficiency.
As it is clearly seen, blending conventional hydrocarbon fuels with NH3 is a rising area of research and overcomes most of the shortcomings of pure ammonia flames. One such hydrocarbon fuel which requires attention in recent times is Liquefied Petroleum Gas (LPG). It finds a variety of uses in domestic and industrial areas owing to its efficiency, portability, versatility and clean burning characteristics compared to other hydrocarbon fuels. In industries, it is used for metal processing, firing of ceramic products, drying and steaming in textile industries, powering automotive vehicles, etc. In the domestic sphere, LPG is primarily used in cooking and in heating devices. In India, LPG is the most commonly used fuel for cooking. However, there are no studies related to flame stability of ammonia-LPG blended flames available in the literature. Therefore, this study initiates an experimental investigation on flame stability for different amounts (% by volume) of NH3 (0, 50, 70, 75, 80%) blended with commercially available LPG. A newly designed and fabricated single slot Bunsen burner (of inner diameter 17.34 mm) is used to establish canonical laminar diffusion and partially premixed NH3-LPG flames. A series of experiments are conducted using this burner by varying different parameters like (i) mixing ratios of NH3 and LPG in fuel mixture, (ii) power ratings (0.1 to 1.1 kW) and (iii) percentages of partial premixing with air (0% to 30%). A qualitative analysis is done using the flame photographs in order to understand the effect of these parameters on the flame stability. The variation of the measured visible flame heights with respect to aforementioned parameters are also reported. Additionally, the effect of increasing NH3 volume fractions in the fuel mixture on adiabatic flame temperature, NO, CO and CO2 production is also studied using equilibrium calculations and the results are reported.
2. Experimental Methods
In the present study, the flame stability of ammonia and LPG blends is characterized by establishing a canonical diffusion and partially premixed flames in a newly designed and fabricated single slot Bunsen burner. The burner tube made up of stainless steel has internal diameter and length of 17.34 mm and 15 cm, respectively. The inner diameter of the burner is chosen such that the Reynolds’ number for the flows inside the tube are well within the laminar regime. The tube length is selected such that the flow is fully developed as it comes out of the burner. A thickness of 1.83 mm is used to ensure optimal heat dissipation. The burner is provided with four inlets around the periphery of the settling chamber. The photographic image of the Bunsen burner and the schematic of the experimental setup are shown in the Figs. 1.1 and 1.2, respectively. The fuel cylinders of commercial LPG and ammonia (purity 99.99%) supplies fuel to the burner and the flow rates of each fuel are controlled using respective Mass Flow Controllers (MFC1 and MFC2). The outlets of the LPG and ammonia MFCs deliver the required fuel flow rates and are connected directly to a settling (or mixing) chamber through the flexible pneumatic pipes. The air used in the experiments are drawn from the main compressor line. The flow rate of air is controlled using MFC3 before it is allowed to mix with the fuel. All the flow rates of ammonia, LPG and air are regulated using Alicat MFC with 0.5% of reading accuracy. The flame photographs are captured with the help of a high-speed camera (EOS M50 Mark II).
The MFC used in measuring the flow rate of a multicomponent fuel such as LPG has some limitations in handling the different components. Therefore, analogous fuel compound has to be chosen based on the molecular weight, viscosity and the major component present in the actual fuel. The molar composition of LPG used in the experiments is measured using a gas chromatography (Model: Perkin Elmer Clarus 580; Asset ID: 8819). It consists of the following components by volume: 44.82% C3H8, 33.27% n-C4H10, 16.82% iso-C4H10, 1.75% iso-C4H8, 1.06% trans-C4H8, 0.33% C3H6, 0.25% iso-C5H12 and 0.082% CH4. The equivalent molecular weight and (lower) calorific value of LPG thus calculated is 51.58 g/mole and 45895.41 kJ/kg, respectively. The viscosity of this multi-component fuel, i.e., LPG, calculated using Wilke’s formula [21] is found to be 7.79 x 10-6 Pa-s. As the calculated molecular weight and viscosity of LPG (ƞLPG/ƞbutane = 1.06) is closer to butane, LPG flow rate in MFC 2 is therefore controlled by setting the fuel as n-butane.
The relationship between the desired flow rate of LPG and the indicated flow rate of n-butane on the MFC display is given by the relationship (Eq. (1)) [22],
Qbutane = QLPG x (ƞLPG/ƞbutane) (1)
where, Q = volume flow rate, and ƞ = viscosity of gas.
The gas set for the other two MFCs is the same as the fluid passing through them. The calorific value and viscosity of ammonia are 18601 kJ/kg [23] and 10.083 x 10-6 Pa-s [23], respectively. LPG and ammonia are blended together to form fuel mixtures of various compositions by volume as shown in Table 1. For each fuel mixture composition, the diffusion and partially
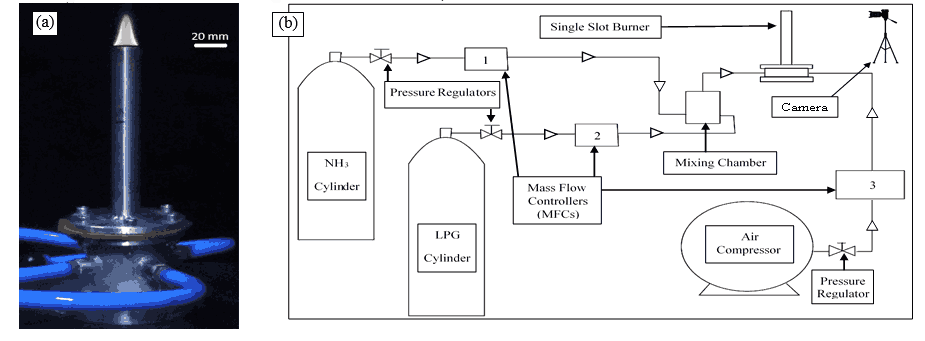
Table 1: Experimental Configurations
|
Burner Inlet & Ambient Conditions |
Fuel Compositions |
Power Rating (kW) |
Flame configurations |
|
|
Temperature: 298 K Pressure: 1 atm |
% vol. NH3 |
% vol. LPG |
0.1, 0.2, 0.3, 0.4, 0.6, 0.8, 1.1 |
(a) Diffusion |
|
0 |
100 |
(b) Partially Premixed (i) 10% (ii) 20% (iii) 30% of stoichio- metric air. |
||
|
50 |
50 |
|||
|
70 |
30 |
|||
|
75 |
25 |
|||
|
80 |
20 |
|||
To set up a flame for each trial, LPG is passed initially through MFC 2 at the required flow rate and a flame is established. Then, ammonia and air (if required) are passed through MFC 1 and 3, respectively at the required flow rates. The photographic images are taken once the initial disturbances in the flame caused by the flow are removed. In each trial, photographic images of the flames are taken for a span of 120 seconds, with the help of a high-speed camera. In each second, 50 consecutive images were captured at regular intervals, leading to 6000 images. A developed MATLAB code is used to retrieve the average flame height from each of these frames in pixels. This flame length is then converted in the units of millimetres from a suitable reference image. A minimum of three such trials are performed for each case. Apart from flame height, the nature and stability of flames are also observed and categorised.
3. Results and Discussion
The effect of power rating, ammonia percentage in fuel mixture and percentage of partial premixing on the flame stability of NH3-LPG flames is analysed qualitatively with the help of photographic flame images. A flame stability map is then developed based on the collective observations obtained from the series of experiments to demarcate the various stability regimes.
3.1. Effect of Ammonia Percentages in Fuel mixture
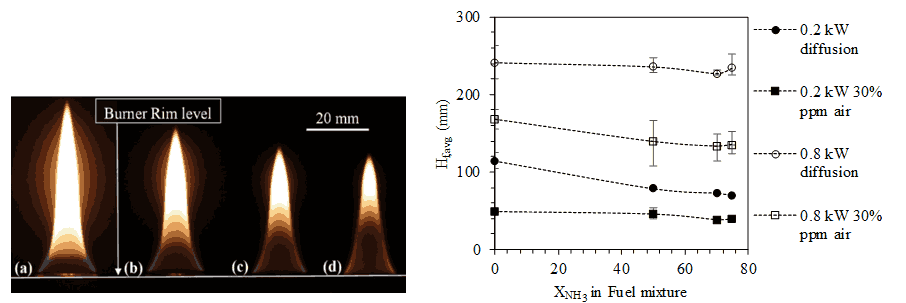
The effect of varying ammonia volume percentage is studied by establishing a diffusion and partially premixed flames with constant power ratings of 0.1, 0.2 and 0.8 kW. The typical flame photographs of 0.1 kW diffusion flame and variation of flame height with respect to ammonia volume percentages are shown in the Figs. 2(i) and 2(ii), respectively. Two primary effects are observed as shown in Figure 2.(i),— (a) a reduction in flame height (~11% for 0.1 kW diffusion flames when 0.8 mole fraction of ammonia in fuel mixture as compared to pure LPG flames) and (b) a decrease in the sooty or luminous zone of the flame. The second observation is promising as it indicates production of less soot with increasing ammonia mole fraction in fuel mixture. A steady flame can be established on a single slot burner with ammonia volume percentage as high as 80% in the fuel mixture provides scope for future steady measurements of temperature and emissions of the flames. Also, the flames being diffusion flames ensure that they are closer to real life domestic applications. It is noted from Figure 2.(ii) that for lower power ratings like 0.2 kW, ammonia causes a higher decrease in flame height for diffusion cases (~39%) than partially premixed cases (~18.7% for 30% partially premixed). However, on increasing power rating (to 0.8 kW in this case), a larger decrease in flame height on ammonia addition is observed for partially premixed cases (~19.9% for 30% partially premixed) than compared to corresponding diffusion cases (~2.94%).
3.2. Effect of Power Rating
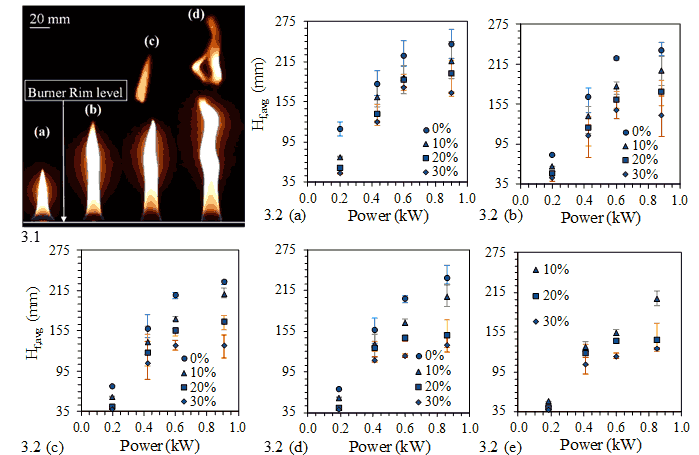
The effect of increasing power by keeping the fuel mixture composition constant at 50:50 by volume for diffusion cases is seen (Figure 3.1). An increase in flame height by approximately 200% is observed as power rating is increased from 0.1 kW to 0.8 kW. However, the flame moves from a momentum driven flow regime to a buoyancy driven flow regime. As a result, the flame tip starts oscillating as we go from 0.1 to 0.2 kW power rating. Thus, the flame is no more steady. Similarly, a further increase in power rating to 0.4 kW takes the flame into a zone where vortex shedding is observed. This phenomenon is even more pronounced when 0.8 kW flames are observed.
The time averaged visible flame heights are experimentally measured using the help of a camera for different compositions of fuel mixture, power ratings and percentages of partial premixing. For a given fuel mixture composition, they are plotted (Figure 3.2 (a)-(e)) against different power ratings and percentages of partial premixing. The average flame length increases with power rating when fuel mixture composition and percentage of partial premixing remains the same. It can also be noted that the increase in flame length with power rating is more pronounced for diffusion cases than partially premixed cases. For example, in Figure 3.2(e), the flame height increases by approximately 300% for 10% partially premixed cases as compared to 240% for 30% partially premixed cases.
3.2.a Error Analysis For Flame Length
Let D= diameter of the burner in mm, P1= pixel co-ordinate of the left end of the burner rim in the flame image, P2=pixel co-ordinate of the right end of the burner rim in the flame image (P2>P1), therefore, (P2-P1) gives us the diameter of the burner in pixels. L=length of the flame in pixels as computed by the MATLAB code. Now, length of the flame is given as,
Lf= D x L/(P2-P1)
Hence, using the theory of propagation of uncertainty [26], uncertainty of flame length is given as,
ΔLf
= ![]() ;
;
such that, δD = uncertainty in mm associated with the measurement of the burner diameter, δP1 = uncertainty in pixels associated with determining pixel co-ordinate of the left end of the burner rim in the flame image and δP2 = uncertainty in pixels associated with determining pixel co-ordinate of the right end of the burner rim in the flame image. uncertainty in mm associated with the measurement of the burner diameter, P1 = uncertainty in pixels associated with determining pixel co-ordinate of the left end of the burner rim in the flame image and P2 = uncertainty in pixels associated with determining pixel co-ordinate of the right end of the burner rim in the flame image.
Taking typical values as the following: D=17.34 mm, δD=0.02 mm and δP1=δP2=2 pixels (estimated from previous trials).
Since ![]()
the final expression for ΔLf (mm) ≈![]() (2)
(2)
Values of P1 and P2 depend on the framing of the video. Attempts are made to keep the value of (P2-P1) as high as possible for a flame of given configuration to ensure higher image quality. The value of L varies from one flame configuration to another.
Sample Calculation
Let us consider the flame of 0.8 kW power rating, 30% of stoichiometric (partially premixed) air with 80:20 NH3:LPG ratio by volume. The values of ‘L’ and (P2-P1) for the three trials taken during flame-length measurement be as follows:
Table 2: Error analysis for a flame-length measurement
|
Trial no. |
Flame-length in pixels (L) |
Diameter of burner in pixels (P2-P1) |
|
1 |
369.05 |
60 |
|
2 |
331.57 |
53 |
|
3 |
218.167 |
35 |
Experimentally,
average flame length = 130.481 mm,
and ΔLf, experimental = 2.2 mm.
Based on error analysis,
For trial 1, ΔLf,1 =![]() =
= ![]() = 7.11 mm
= 7.11 mm
For trial 2, ΔLf,2 =![]() =
= ![]() = 8.187 mm
= 8.187 mm
For trial 3,ΔLf,2 =![]() =
= ![]() = 8.187 mm
= 8.187 mm
Clearly, ΔLf, experimental < Average of ΔLf,1, ΔLf,2 and ΔLf,3.
However, there are measurements where the experimental uncertainty is more than the theoretically calculated uncertainty. This can be attributed to other sources of uncertainties and sources of errors while carrying out these experiments:
- Disturbances in the flow pattern of the ambient air which affects the flame structure directly. This effect is more pronounced in diffusion flames as they are entirely dependent on ambient air for combustion.
- Non-homogeneity in the LPG mixture of gases.
- Changes in ambient conditions (temperature, pressure, humidity, etc.) depending on the weather on the given day of experiments.
3.3. Effect of Partial Premixing with air
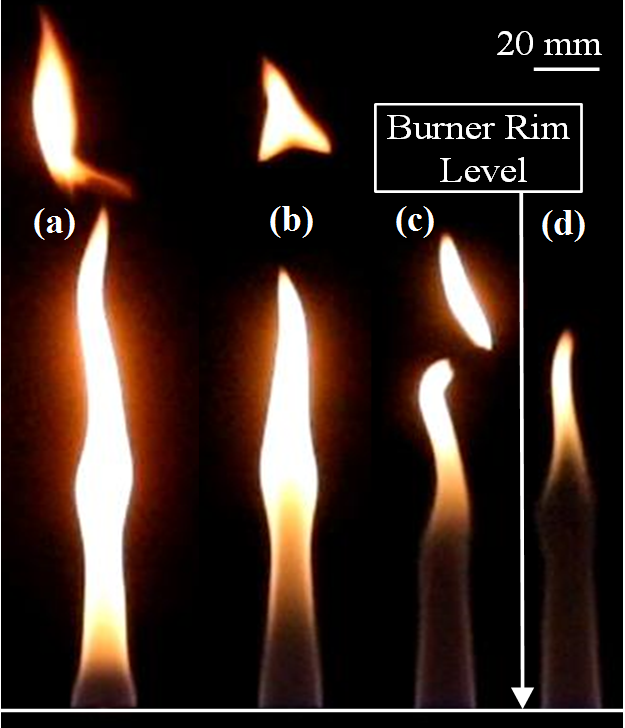
The effect of partial premixing with air on LPG-NH3 flames are observed at a constant power rating of 0.6 kW and composition of 50-50 fuel mixture. As the extent of partial premixing increases from 0% to 30% of stoichiometric air (Figure 4.1 and Figure 3.2 (b)), the average flame length decreases by approximately 39%. The increase in availability of air leads to burning of the fuel mixture over a shorter distance from the burner rim. There exist luminous regions on top of these flames due to soot formation, the extent of which reduces with increase in the percentage of premixed air (Figure 4.1). Beneath these luminous sooty regions, there exist non-luminous regions which are associated with combustion closer to stoichiometry. The extent of these non-luminous zones increases despite the decrease in flame heights.
A transition in tendency of flames with vortex shedding to flames with oscillating tips with increase in air flow rate is also observed. Figure 4.2 shows that for a given fuel composition, the transition of flames from regimes of vortex shedding to regimes of oscillating tip happen at lower extents of partial premixing when the power rating is low. These qualitative observations on increasing the extent of partial premixing hold true for flames with different power ratings and compositions of fuel mixtures.
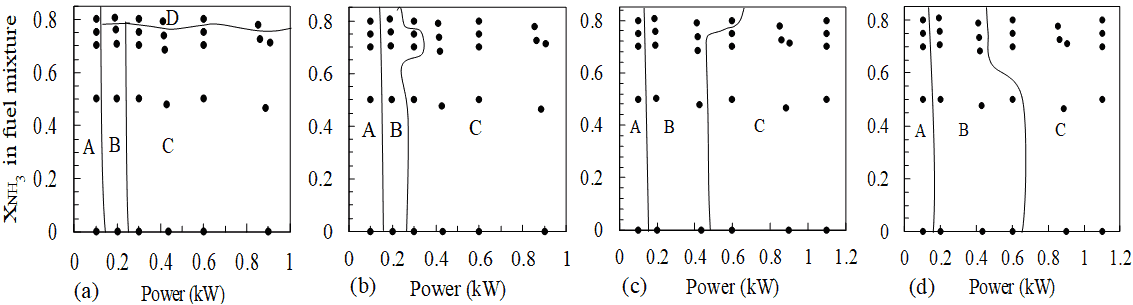
3.4. Stability Maps
Different regimes (steady, oscillating tips and vortex shedding) of flames are observed for different combinations of power rating, fuel mixture composition and degrees of partial premixing with air. Thus, it is important to develop a stability map (shown in Figure 5) which demarcates the stability regime in which the flame
 (a)
(a)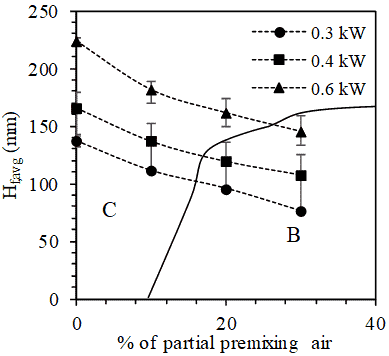 (a)
(a)lies for a particular combination of the aforementioned parameters. From Figure 5, it is observed that the increase in percentage of partial premixing has a stabilising effect on flames. For example, as shown in Figure 5 (a), fuel mixture with 0.8 mole fraction of NH3 exhibits lift off (regime D). But this flame transitions into a vortex shedding regime (C) on partial premixing. Also, for any given fuel composition and percentage of partial premixing with air, an increase in power rating increases the tendency of the flame to transition from the regime of oscillating tip to that of vortex shedding. It is interesting to note that for 10% and 20% partially premixed cases (Figure 5(b)&(c)), flames with oscillating tip can be found for higher concentrations of NH3 in fuel mixtures whereas for 30% partially premixed case, the same is found at lower concentrations of NH3.
3.5. Equilibrium Calculations
In this study, the stoichiometric equations for the combustion of the 5 different compositions (namely, 0:100, 50:50, 70:30, 75:25 and 80:20 ratios of NH3 and LPG by volume) of fuel mixtures are estimated by equilibrium calculations using the software GasEQ [24]. A constant pressure adiabatic process was considered. The probable product species considered were N2, O2, CO, CO2, H2O, NO, H2, H, OH, O and N. This was done on the basis of the concept of major and minor species [25]. However, the significant products turned out to be N2, O2, CO, CO2, H2O and NO.
It is seen that the number of moles of stoichiometric air required for the complete combustion of the fuel mixture decreases as the mole fraction of NH3 increases in fuel mixture. As shown in Figure 6.1, at constant pressure for one mole of these fuel mixtures, on increasing NH3 mole fraction, a reduction in adiabatic flame temperature is observed. This is accompanied by a drop in CO and CO2 mole fractions in the product mixture (Figure 6.2) because of decreasing concentrations of hydrocarbon (LPG) in the fuel mixture. However, the mole fraction of NO (the most dominant contributor to NOx emissions) remains the same even on increasing mole fraction of NH3 in fuel mixture. This is interesting because although NO production is enhanced by NH3 addition due to N atom contribution in the combustion zone, NO production is also found to be highly sensitive to temperature (thermal NO) which decreases on increasing NH3 mole fraction in the fuel mixture.
4. Conclusion
Ammonia is considered to be a good alternative for traditional hydrocarbon fuels in order to mitigate the global warming caused by greenhouse gases. However, for NH3 to be a practical substitute, it must be blended with traditional fuels due to its lower laminar flame speed and reactivity when compared to those of conventional hydrocarbon fuels. In this work, an experimental investigation is carried out using NH3-LPG blends in varying proportions for different power ratings
 (a)
(a)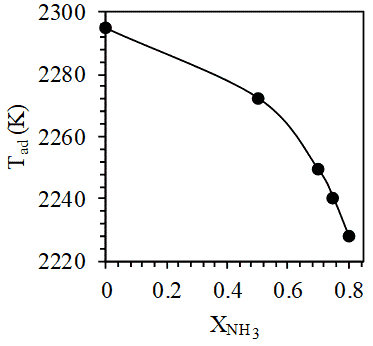 (a)
(a)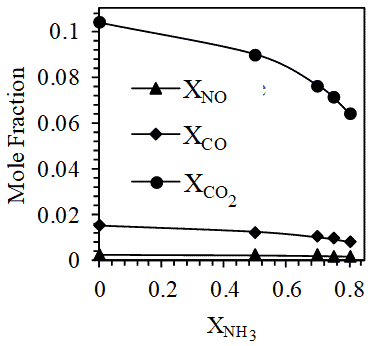 (a)
(a)(0.1-1.1 kW) by establishing both diffusion and partially premixed (10%, 20% and 30%) flames in a newly constructed single slot burner. The important findings from the study are as follows,
(i) Steady diffusion flames of lower power rating 0.1 kW are established with varying NH3 mole fraction till as high as 0.8 in fuel mixture. It is observed that on increasing NH3 in the fuel mixture for steady flames, there is a decrease in flame length, shortening of the luminous sooty zone and an increase in length of the non-luminous zone.
(ii) Stability maps are plotted for different configurations of fuel compositions, power ratings and percentages of partial premixing. This identifies four stability regimes among flames—(A) steady flames, (B) flames with oscillating tip, (C) flames with vortex shedding and, (D) unstable flames with lift-off. As power rating is increased, flames transition from being at regimes A to B and finally C. All diffusion flames except for 0.1 kW, with 80% ammonia (by volume) in the fuel mixture lie in regime D.
(iii) From stability maps, it is inferred that there is a tendency of the flames to undergo transition from regime (C) to regime (B) as the extent of partial premixing increases.
(iv) Flames in regime B are found for larger concentrations of NH3 in fuel mixtures for 10% and 20% partially premixed instances, whereas the same can be found for lower concentrations of NH3 for 30% partially premixed case.
(v) Flame lengths are found to increase with increase in power rating and decrease with increase in percentage of partial premixing with air.
(vi) At constant pressure for one mole of fuel mixture, the adiabatic flame temperature, mole fractions of CO and CO2 in product mixture decreases with increase in mole fraction NH3 in the fuel mixture.
(vii) NO is the primary contributor to NOx emissions. However, for one mole of fuel mixture under constant pressure, an increase in NH3 mole fraction in fuel does not produce drastic changes in NO mole fraction among products. This is because the effect of increasing the mole fraction of N atom containing NH3 is nullified by the decrease in adiabatic flame temperature since NO formation is highly dependent on temperature (thermal NO).
5. Acknowledgements
The authors would like to thank the Indian Institute of Technology, Madras and Department of Science and Technology, India (sanction no. CRG/2022/007485) for providing the infrastructure to carry out the study. We are also grateful to Mr. Vinoth for his immense help during the experiments.
References
[1] Yun Ge, "Experimental and numerical investigation of combustion characteristics of carbon-free NH3/H2 blends in N2O,"Int J. Hydrogen Energy, vol. 49, part B, pp. 510-520, 2024. View Article
[2] Z. A. Shah, "A review of recent studies and emerging trends in plasma-assisted combustion of ammonia as an effective hydrogen carrier," Int J. Hydrogen Energy, vol. 51, part D, pp. 354-374, 2024. View Article
[3] H. Mikulcic, "Numerical simulation of ammonia/methane/air combustion using reduced chemical kinetics models," Int J. Hydrogen Energy, vol. 46, no. 45, pp. 23548-23563, 2021. View Article
[4] M. Ilbas, "Oxidizer effects on ammonia combustion using a generated non-premixed burner," Int J. Hydrogen Energy, vol. 47, no. 24, pp. 12317-12337, 2022. View Article
[5] R. Weber, "High temperature air combustion (HiTAC): how it all started for applications in industrial furnaces and future prospects," Applied Energy, vol. 278, pp. 115551, 2020. View Article
[6] S. Wang, "Experimental study and kinetic analysis of the laminar burning velocity of NH3/syngas/air, NH3/CO/air and NH3/H2/air premixed fames at elevated pressures," Combustion and Flame, vol. 221, pp. 270-287, 2020. View Article
[7] T. Cai, "Temperature dependence of laminar burning velocity in ammonia/dimethyl ether-air premixed flames," J. of Thermal Science, vol. 31, pp. 187-197, 2022. View Article
[8] J. H. Lee, "Studies on properties of laminar premixed hydrogen-added ammonia/air flames for hydrogen production," Int J. Hydrogen Energy, vol. 35, no. 3, pp. 1054-1064, 2010. View Article
[9] X. Han, "Experimental and kinetic modelling study of laminar burning velocities of NH3/air, NH3/H2/air, NH3/CO/air and NH3/CH4/air premixed fames," Combustion and Flame, vol. 206, pp. 214-226, 2019. View Article
[10] C. Lhuillier, "Experimental investigation on laminar burning velocities of ammonia/hydrogen/air mixtures at elevated temperatures," Fuel, vol. 263, pp. 116653, 2020. View Article
[11] J. Li, "Study on using hydrogen and ammonia as fuels: Combustion characteristics and NOx formation," International J.of Energy Research, vol. 38, no. 9, pp. 1214-1223, 2014. View Article
[12] J. Li, "Numerical study on laminar burning velocity and ignition delay time of ammonia flame with hydrogen addition," Energy, vol. 126, pp. 796-809, 2017. View Article
[13] E. C. Okafor, "Measurement and modelling of the laminar burning velocity of methane-ammonia-air flames at high pressures using a reduced reaction mechanism," Combustion and Flame, vol. 204, pp. 162-175, 2019. View Article
[14] J. Ku, "Propagation and emissions of premixed methane-ammonia/air flames," Energy, vol. 201, pp. 117632, 2020. View Article
[15] T. Shu, "An experimental study of laminar ammonia/methane/air premixed fames using expanding spherical fames," Fuel, vol. 290, pp. 120003, 2021. View Article
[16] M. L. Lavadera, "Comparative Effect of Ammonia Addition on the Laminar Burning Velocities of Methane, n-Heptane, and Iso-octane," Energy and Fuels, vol. 35, no. 9, pp. 7156-7168, 2021. View Article
[17] S. Lui, "Experimental and numerical study of laminar fame speeds of CH4/NH3 mixtures under oxy-fuel combustion," Energy, vol. 35, pp. 250-258, 2019. View Article
[18] M. Martin, "Optimal year-round production of DME from CO2 and water using renewable energy," J. of CO2 Utilization, vol. 3, pp. 105-113, 2016. View Article
[19] P. Ronan, "Laminar fame speed of ethanol/ammonia blends- An experimental and kinetic study," Fuel Communications, vol. 10, pp. 100052, 2022. View Article
[20] Z. Wang, "Experimental and kinetic study on the laminar burning velocities of NH3 mixing with CH3OH and C2H5OH in pre mixed fames," Combustion and Flame, vol. 229, pp. 111392, 2021. View Article
[21] B. E. Poling, J. M. Prausnitz and J. P. O'Connell, The properties of gases and liquids. McGraw-Hill Professional, 2000. View Article
[22] Alicat Scientific [Online]. Available: View Article
[23] E. W. Lemmon (2018), National Institute of Standards and Technology, US Department of Commerce [Online]. Available: View Article
[24] Chris Morley (2005) [Online]. Available: View Article
[25] S. R. Turns, An Introduction to Combustion, Concepts and Applications. McGraw-Hill Professional, 2000.
[26] J. R. Taylor, An Introduction to error analysis The Study of Uncertainties in Physical Measurements. University Science Books, 1997.

